Radioactive Decay - Risk Management Services
advertisement

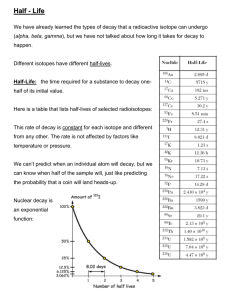
Health, Safety and Environment www.hse.ubc.ca Radioactive Decay The rate of radioactive decay is referred to as the activity: measure of the number of radionuclide decays per unit of time UNITS OF ACTIVITY Becquerel (Bq) = 1 disintegration per second (dps) 1 Bq = 27 picoCuries 1 kiloBq (kBq) = 27 nanoCuries 1 MegaBq (MBq) = 27 microCuries (µCi) 1 GigaBq (GBq) = 27 milliCuries (mCi) 1 TeraBq (TBq) = 27 Curies *The rate of decay of a radionuclide is exponential with time. At time T, activity A is given by: A = Aoe –λT A =initial activity o λ = decay constant Activity of 1 mg of U-238 is 10 Bq Activity of 1 mg of Am-241 is 1 x 108 Bq ????? The difference is related to a unique property of all radionuclides called the half-life. Half-Life *The half-life of a radionuclide is the time required for it to lose 50% of its activity by radioactive decay. *Each radionuclide has its own unique half-life regardless of the quantity or form – solid,gas,liquid. * The half-life is an unalterable property of the radioisotope. * Half-lives of radioisotopes vary: Uranium-238 4.5 x 109 years Americium-241 4.32 x102 years Polonium-212 0.3 microseconds •After 7 half-lives the activity decreases to about 1% of its original value. •After 10 half-lives the activity reduces to one thousandth of its original value. Mathematically the concept of half-life Can be defined by : A = Ao ____ n= #half-lives 2n The decay constant and half life are related : t1/2 = ln(2) λ = 0.693 λ t1/2 = 0.693 λ A = Aoe –λT Combine to form: A= / -0.693 t T1/2 Aoe A researcher has 500 MBq of use it for 35 days. 32P but cannot What is the activity when she wants to use the material? For 32P T ½ = 14.3 days A = Ao 2n After 1 half-life (n=1) A = 500 2 = 250 MBq After 2 half-lives (n=2) A = 500 4 = 125 MBq After 3 half-lives (n=3) A = 500 8 = 62.5 MBq A= / -0.693 t T1/2 Aoe A0 500 MBq , t 35 d, T1/2 14.3 days A 500MBq x e -.693 x 35/14.3 500MBq x e A = 91.7 MBq -1.696 *History *Radiation : types alpha, beta, positron, gamma, x-rays neutron activation *Energy levels: ionizing radiation. *Radioactive decay For EXAM! Learn characteristics of two isotopes •Type of radiation : alpha, beta, gamma •Energy of radiation: eV •Shielding required: material and thickness •Half-life (Tables 1, 4 of manual)