Nuclear Chemistry
advertisement
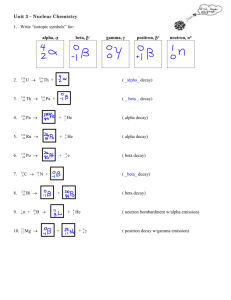
Nuclear Chemistry Chapter 25 What do you think of when you hear Nuclear Chemistry? Henri Becquerel (1852-1908) ◦ Originally thought sunlight caused uranium to radiate ◦ Discovered the spontaneous radiation while waiting for a sunny day to test his theory Marie Curie (1867-1934) and her husband Pierre Curie (1859-1906) ◦ Worked w/ Becquerel ◦ Eventually showed that the rays were from uranium atoms ◦ Came up with the term “radioactivity” to describe History Some isotopes are stable and others are not. ◦ Nucleus is protons and neutrons Protons are positive so they repel Neutrons are only stable when near a proton ◦ Some have: Too many protons Too many neutrons Just plain too many ◦ So they “fall apart” Why Radioactive? First 3 kinds of radiation found First 3 kinds of radiation found ALPHA ◦ Positive matter ◦ Exactly like a Helium nucleus BETA ◦ Negative matter ◦ Exactly like an electron GAMMA ◦ High energy wave ◦ NOT matter so no charge Summary of Radiation Alpha Radiation- when a helium nuclei has been emitted from a radioactive source. Types of Radiation Beta Radiation – An electron resulting from the breaking apart of a neutron in an atom. Types of Radiation Gamma Radiation – a high-energy photon emitted by a radioisotope. (electromagnetic radiation) Extremely Dangerous! Types of Radiation Type of Radiation Symbol Alpha 𝛼 Beta 𝛽 Gamma 𝛾 Mass Nuclear Charge Particle 4 amu 2+ 4 2𝐻𝑒 1- 𝑒− 0 𝛾 1 1840 amu 0 Look for patterns Try to explain the patterns Test your ideas with known things Use the pattern to figure out unknown things Adjust as necessary! How can we use what we know? The blue ones have at least one stable isotope. Others do not! http://catalog.flatworldknowledge.com Chart helps predict type of decay ◦ Too many neutrons? Emit a beta particle to change a neutron to a proton and move closer to the band of stability ◦ Just too big? Emit an alpha particle to reduce size of nucleus There are many types of decay, but chart helps give us targets when trying to manipulate elements to do what we want How does this help? Mass numbers and charges are conserved (equal on both sides) Example Alpha Decay ◦ ◦ ◦ ◦ ◦ ◦ ◦ 238 94𝑃𝑢 → 𝐴𝑍𝑋 + 42𝐻𝑒 Mass number of product A= ? Atomic Number of product Z= ? Reaction Product X = ? 238= A+4 94=Z+2 So… 234 92𝑈 is the answer Writing and Balancing Nuclear Equations Mass numbers and charges are conserved (equal on both sides) Example Beta Decay 238 94𝑃𝑢 → 𝐴𝑍𝑋 + 𝑒 − ( −10𝛽) Mass number of product A= ? Atomic Number of product Z= ? Reaction Product X = ? since beta decay is the break up of a neutron into a proton and an electron the mass # doesn’t change ◦ Z will be one greater Z= 95 ◦ So… 238 95𝐴𝑚 is the answer ◦ ◦ ◦ ◦ ◦ Writing and Balancing Nuclear Equations Show the products of Bismuth -212 undergoing an alpha decay 212 83𝐵𝑖 → 42𝐻𝑒 + 208 81𝑇𝑙 Now the Daughter thallium undergoes a beta decay 208 81𝑇𝑙 → 𝑒 − ( −10𝛽) + Practice 208 82𝑃𝑏 Half-Life is the time required for half of the atoms in a radioactive sample to decay Number of half-lives Elapsed time Amount of strontium-90 present 0 0y 10.0g 1 29 y 5.00g 2 58 y 2.50g 3 87 y 1.25g 4 116 y 0.625g Half -Life Practice Bandages can be sterilized by exposure to gamma radiation from colbalt-60, which has a half-life of 5.27 years. How much of a 10.0 mg sample of cobalt-60 is left after one half-life? Two half-lives? Three halflives? How many years is 3 half-lives? 5.00mg; 2.50mg; 1.25mg; 15.81y Half-Life