Radioactive Decay - Kalaheo High School
advertisement
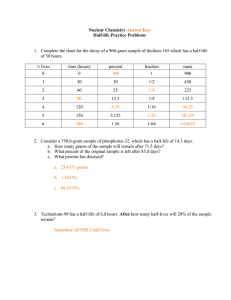
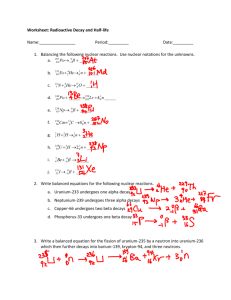
Section 3: Radioactive Decay and Half-Life The spontaneous emission of rays or particles from certain nuclei as they “decay,” such as Uranium. These rays or particles are called nuclear radiation. They come in three types: Alpha Radiation: Helium nucleus; 2+ Charge; Low power (0.05 mm body tissue) Beta Radiation: electron; 1- Charge; Moderate power (4 mm body tissue) Gamma Radiation: electromagnetic wave; neutral charge; High Power (penetrates entire body easily) While the process is spontaneous, it is not instantaneous… it takes time! The time required for HALF of the atoms of a radioactive substance to decay is called a half-life. Fluorine 18 9 18 ( F) has a half-life of 110 seconds. This material is used extensively in medicine. The hospital laboratory begins the day with 10 18 grams of 9 F… 1 half-life = 110 seconds 660 seconds = 6 half-lives 110 seconds 6 1) 2) 3) 4) 5) 6) half-lives = 10 divided in half, 6 times 10/2 = 5 grams 5/2 = 2.5 grams 2.5/2 = 1.25 grams 1.25/2 = 0.625 grams 0.625/2 = 0.3125 grams 0.3125/2 = 0.15625 grams After 11 minutes, only 0.16 g of the 10 g sample remain!!!!!!