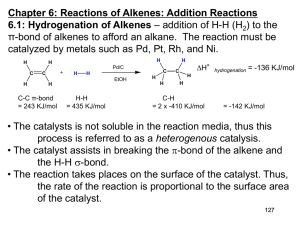
Alkene-Addn-PartB-2012-ques
advertisement

Acid-Catalyzed Hydration of Alkenes Acid-Catalyzed Hydration of Alkenes C C + H—OH H C C OH reaction is acid catalyzed; typical hydration medium is 50% H2SO4-50% H2O Follows Markovnikov's Rule Hydration – Thermodynamics Hydrohalogenation & hydration reactions are at equilibrium. Hydration – Thermodynamics How could Le Châtelier’s principle be used to shift the equilibrium to the right or left? SEE: CONCEPTUAL CHECKPOINT 9.11. Hydration – Thermodynamics Similar to hydrohalogenation, the stereochemistry of hydration reactions is controlled by the geometry of the carbocation. Draw the complete mechanism for the reaction above to show WHY a racemic mixture is formed. SEE: SKILLBUILDER 9.3. Markovnikov's Rule H3C C H3C CH3 H 50% H2SO4 C CH3 50% H2O CH3 C CH2CH3 OH (90%) Follows Markovnikov's Rule CH2 50% H2SO4 CH3 50% H2O OH (80%) Mechanism involves a carbocation intermediate is the reverse of acid-catalyzed dehydration of alcohols to alkenes H3C H+ C H3C CH3 CH2 + H2O CH3 C OH CH3 Addition of Water to Alkene (alcohols) Addition of Water to Alkenes (alcohols) Question Which alkene will undergo acid-catalyzed hydrolysis at the fastest rate? A) B) C) D) Principle of Microscopic Reversibility H3C H+ C H3C CH3 CH2 + H2O CH3 C CH3 OH In an equilibrium process, the same intermediates and transition states are encountered in the forward direction and the reverse, but in the opposite order. H2O addition: Markovnikov's Rule Question The product isolated from the acid-catalyzed hydration of (Z)-3-methyl-2-pentene is: A) 2-ethyl-2-butanol B) 2-ethyl-1-butanol C) 3-methyl-2-pentanol D) 3-methyl-3-pentanol Question The product isolated from the acid-catalyzed hydration of (E)-3-methyl-2-pentene is: A) chiral B) achiral Oxymercuration-Demercuration Because rearrangements often produce a mixture of products, the synthetic utility of Markovnikov hydration reactions is somewhat limited. OXYMERCURATION-DEMERCURATION is an alternative process that can yield Markovnikov products avoiding rearrangements Oxymercuration-Demercuration OXYMERCURATION begins with mercuric acetate. How would you classify the mercuric cation? As a nucleophile or an electrophile? As a Lewis acid or Lewis base? Oxymercuration-Demercuration Similar to how we saw the alkene attack a proton previously, it can also attack the mercuric cation. Resonance stabilizes the mercurinium ion. Can you draw a reasonable resonance hybrid? Oxymercuration-Demercuration The mercurinium ion is also a good electrophile, and it can easily be attacked by a nucleophile, even a weak nucleophile such as water. NaBH4 is generally used to replace the –HgOAc group with a –H group via a free radical mechanism. Addition of Alcohol to Alkenes (ethers) Stereochemistry of Addition to Alkenes C C + E—Y E C C Y Regiochemistry withstanding, in order to understand the stereochemistry of the product, you must consider two things: (1) Stereochemistry of the starting alkene (cis or trans; Z or E) (2) Stereochemistry of the addition (syn or anti) Stereochemistry of Addition to Alkenes C C + E—Y E C C Y Optically inactive reactants produce optically inactive products. (Racemic mixtures are optically inactive) The correlary is that an optically active starting material MAY produce an optically active product depending on the mechanism. Question The product isolated from the acid-catalyzed hydration of (E)- or (Z)-3-methyl-2-pentene is: A) optically active B) an optically inactive racemic mixture C) an optically inactive enantiomer Hydroboration-Oxidation of Alkenes Hydroboration-Oxidation To achieve anti-Markovnikov hydration, hydroboration-oxidation is often used. Note that the process occurs in two steps. Synthesis Suppose you wanted to prepare 1-decanol from 1-decene? OH Needed: a method for hydration of alkenes with a regioselectivity opposite to Markovnikov's rule. Synthesis Two-step reaction sequence called hydroborationoxidation converts alkenes to alcohols with a regiochemistry opposite to Markovnikov's rule. 1. hydroboration 2. oxidation OH Hydroboration Step C C + H—BH2 H C C BH2 Hydroboration can be viewed as the addition of borane (BH3) to the double bond. But BH3 is not the reagent actually used. Hydroboration Step C C + H—BH2 H C C BH2 Hydroboration reagents: H H2B BH2 H Diborane (B2H6) normally used in an ether-like solvent called "diglyme" Hydroboration Step C C + H H—BH2 C C Hydroboration reagents: Borane-tetrahydrofuran complex (H3B-THF) •• +O – BH 3 BH2 Oxidation Step H2O2, HO– H C C BH2 H C C Organoborane formed in the hydroboration step is oxidized with hydrogen peroxide. OH Example 1. B2H6, diglyme 2. H2O2, HO– OH (93%) Example H3C CH3 C H3C C H 1. H3B-THF 2. H2O2, HO– CH3 H OH C C CH3 H (98%) CH3 Features of Hydroboration-Oxidation hydration of alkenes regioselectivity opposite to Markovnikov's rule no rearrangement stereospecific syn addition Example 1. B2H6, diglyme OH 2. H2O2, HO– (82%) Stereochemistry of Hydroboration-Oxidation Features of Hydroboration-Oxidation hydration of alkenes regioselectivity opposite to Markovnikov's rule no rearrangement stereospecific syn addition syn Addition H and OH become attached to same face of double bond H CH3 1. B2H6 2. H2O2, NaOH H CH3 HO H only product is trans-2-methylcyclopentanol (86%) yield Question Hydroboration-oxidation of which one of the following yields a primary alcohol as the major product? A) B) C) D) Question H3C CH3 C H3C C H 1. H3B-THF 2. H2O2, HO– CH3 H OH C C CH3 H A) The product is achiral B) The product is optically active C) The product is a racemic mixture D) The product is a single enantiomer CH3 Conversion of Alkenes to Vicinal Halohydrins Addition of Halogens in the Presence of Water (halohydrins) C C + X2 X C C X alkenes react with X2 to form vicinal dihalides C C + X2 X C X C alkenes react with X2 to form vicinal dihalides alkenes react with X2 in water to give vicinal halohydrins C C + X2 + H2O X C C OH + H—X Examples H2O H2C CH2 + Br2 BrCH2CH2OH (70%) H H Cl2 OH H2O H Cl H anti addition: only product Examples H2O H2C CH2 + Br2 BrCH2CH2OH (70%) H H Cl2 OH H2O H Cl H anti addition: only product Perspective formula Fischer projection Regioselectivity CH3 H3C C H3C CH2 Br2 H2O CH3 C CH2Br OH (77%) Markovnikov's rule applied to halohydrin formation: the halogen adds to the carbon having the greater number of hydrogens. Explanation H H H .. O .. O H3C H3C C CH2 : Br : H3C H3C CH2 C : Br : transition state for attack of water on bromonium ion has carbocation character; more stable transition state (left) has positive charge on more highly substituted carbon H Question What is the product (in addition to its enantiomer) of the following reaction? B r2, H 2O A. Br C. OH B. OH Br Br OH D. OH Br Conversion of Alkenes to Vicinal Diols Syn Dihydroxylation SYN dihydroxylation adds across the C=C double bond in ONE step. Syn Dihydroxylation MnO41- is similar to OsO4 but more reactive. SYN dihydroxylation occurs with KMnO4 only under mild conditions (cold temperatures). Diols are commonly further oxidized by MnO41-, and in addition MnO41- is reactive toward many other functional groups as well. It is very useful in qualitative analysis due to a pronounced color change. SEE: CONCEPTUAL CHECKPOINT 9.33.