Alkenes, Dienes, Alkynes
advertisement

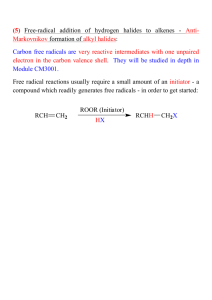
ALKENES Hydrocarbons Aliphatic Alkanes Aromatic Alkenes Alkynes Alkene Nomenclature Alkenes Alkenes are hydrocarbons that contain a carbon-carbon double bond also called "olefins" characterized by molecular formula CnH2n said to be "unsaturated" Alkene Nomenclature H2C CH2 Ethene or Ethylene (both are acceptable IUPAC names) H2C CHCH3 Propene (Propylene is sometimes used but is not an acceptable IUPAC name) Alkene Nomenclature H2C CHCH2CH3 1-Butene 1) Find the longest continuous chain that includes the double bond. 2) Replace the -ane ending of the unbranched alkane having the same number of carbons by -ene. 3) Number the chain in the direction that gives the lowest number to the doubly bonded carbon. Alkene Nomenclature H2C CHCHCH2Br CH3 4) If a substituent is present, identify its position by number. The double bond takes precedence over alkyl groups and halogens when the chain is numbered. The compound shown above is 4-bromo-3-methyl-1-butene. Alkene Nomenclature H2C CHCHCH2OH CH3 4) If a substituent is present, identify its position by number. Hydroxyl groups take precedence over the double bond when the chain is numbered. The compound shown above is 2-methyl-3-buten-1-ol. Alkenyl groups methylene H2C vinyl H2C CH allyl H2C CHCH2 isopropenyl H2C CCH3 Structure of Ethylene bond angles: H-C-H = 117° H-C-C = 121° bond distances: C—H = 110 pm C=C = 134 pm Planar Bonding in Ethylene • Side-by-side overlap of half-filled p orbitals gives a bond Isomers Isomers are different compounds that have the same molecular formula. H CH2CH3 C H 1-Butene H3C H H C C H C H3C H 2-Methylpropene CH3 C H3C H H3C C C H cis-2-Butene H C CH3 trans-2-Butene Stereoisomers H3C CH3 C H H H3C C C H cis-2-Butene H C CH3 trans-2-Butene Stereochemical Notation cis (identical or analogous substituents on same side) trans (identical or analogous substitutents on opposite sides) Reactions of Alkenes The characteristic reaction of alkenes is addition to the double bond. C C + A—B A C C B Hydrogenation of ethylene H C H + C H H H—H H H C C H H H H exothermic H° = –136 kJ/mol catalyzed by finely divided Pt, Pd, Rh, Ni Mechanism of catalytic hydrogenation B Y A C H H X C H H General equation for electrophilic addition C C – + E—Y E C C Y When EY is a hydrogen halide C C – + H—X H C C X Markovnikov’s Rule When an unsymmetrically substituted alkene reacts with a hydrogen halide, the hydrogen adds to the carbon that has the greater number of hydrogen substituents, and the halogen adds to the carbon that has the fewer hydrogen substituents. Markovnikov’s Rule CH3CH2CH CH2 HBr acetic acid CH3CH2CHCH3 Br (80%) Example Acid-Catalyzed Hydration of Alkenes C + C H—OH reaction is acid catalyzed; typical hydration medium is 50% H2SO4-50% H2O H C C OH Cyclopentene +Br2 Bromonium ion – trans-Stereochemistry in vicinal dibromide Epoxidation of Alkenes O C C + RCOOH peroxy acid O C C O + RCOH Polymerization of alkenes cationic polymerization free-radical polymerization coordination polymerization Free-Radical Polymerization of Ethylene H2C CH2 200 °C 2000 atm CH2 CH2 CH2 CH2 O2 peroxides CH2 polyethylene CH2 CH2 Free-Radical Polymerization of Propene H2C CHCH3 CH CH CH CH CH CH CH H CH3 H CH3 H CH3 H polypropylene H2C=CHCl polyvinyl chloride H2C=CHC6H5 polystyrene F2C=CF2 Teflon Classes of Dienes Classification of Dienes isolated diene conjugated diene C cumulated diene Nomenclature (2E,5E)-2,5-heptadiene (2E,4E)-2,4-heptadiene C 3,4-heptadiene Isolated diene bonds are independent of each other p orbitals overlap to give extended bond encompassing four carbons Conjugated diene Cumulated Dienes C C C cumulated dienes are less stable than isolated and conjugated dienes Structure of Allene 118.4° 131 pm linear arrangement of carbons nonplanar geometry 1,2-Addition versus 1,4-Addition 1,2-addition of XY 1,4-addition of XY Y Y X X Alkynes Hydrocarbons Aliphatic Alkanes Aromatic Alkenes Alkynes Nomenclature Acetylene and ethyne are both acceptable IUPAC names for HC CH Higher alkynes are named in much the same way as alkenes except using an -yne suffix instead of -ene. HC CCH3 Propyne (CH3)3CC HC CCH2CH3 1-Butyne CCH3 4,4-Dimethyl-2-pentyne Structure linear geometry for acetylene H 120 pm C C 106 pm CH3 106 pm 121 pm C C 146 pm H H 106 pm Bonds in Acetylene Each carbon is connected to a hydrogen by a bond. The two carbons are connected to each other by a bond and two bonds. Acidity of Acetylene andTerminal Alkynes H C C Reactions of Alkynes Acidity Hydrogenation Metal-Ammonia Reduction Addition of Hydrogen Halides Hydration Addition of Halogens Ozonolysis