36-Alkenes
advertisement

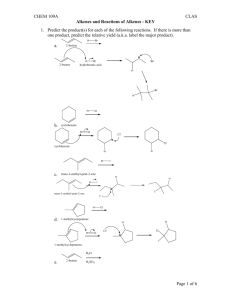
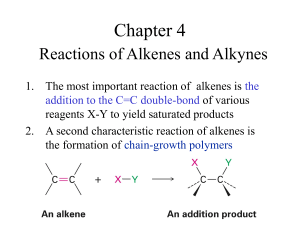
Drill: Name the following: Alkenes Alkenes •Hydrocarbons with at least one double bond Alkenes •Each double bond reduces the number of hydrogens in the hydrocarbon by two Alkenes •All alkenes are unsaturated hydrocarbons Unsaturated Hydrocarbons •Contain less than the maximum number of hydrogens Alkenes Naming Alkenes •Number the carbons on the chain; so that, the double bond has the lowest possible number Naming Alkenes •The double bond will always fall between two numbered carbons Naming Alkenes •Apply the lower of those two numbers to the double bond Naming Alkenes 4 2 1 3 2-butene or Buta-2-ene Problems 4 2 1 3 2 1 3 4 Geometric Isomers 4 2 1 3 2 1 3 4 Geometric Isomers •Isomers whose arrangement is same side (cis) or opposite side (trans) of a stationary bond Naming Alkenes •Draw a line through the double bond parallel to each of the bonds in the double bond Naming Alkenes 4 2 1 3 Naming Alkenes •If the two ends of the chain are on the same side of the line, it is a cis-double bond Naming Alkenes •If the two ends of the chain are on opposite sides of the line, it is a trans-double bond Naming Alkenes •Place cis or trans at the start of the name in the organic compound trans-2-butene trans-buta-2-ene 4 2 1 3 Draw the Following: trans-3-ethyl-4,6dimethyl-5cyclopentyl-2-octene Drill: Draw the condensed, skeletal, & stick structures for: cis-3-ethyl-4,6-dimethyl-5cyclopropyl-2-octene Review Alkenes & Cycloalkanes Name: Main chain Name ending Branches trans-3,4-dimethyl3-hexene Name: Main Chain Name ending Branches trans-3-methyl-2cyclopropyl-3-heptene Name: Main Chain Name Ending Branches cis,trans-7-methyl2,4-nonadiene Name: Main chain or cycle: Branches: 1,1,2,2,4,4hexamethylcyclohexane: Name: Main chain or cycle: Branches: Cyclohexylcyclohexane: Name: Main chain or cycle: Branches: 1,4-dicyclohexyl cyclohexane: Draw: •trans-1cyclobutyl-2methyl-3-hexene Draw: cis-2-methyl-1octylcyclohexene Draw & Name isomers of: •C5H10 Naming Alkenes •Name the alkenes on the board