chapter8 - Department of Chemistry and Biochemistry
advertisement

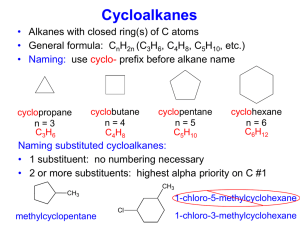
Chapter 8 Alkenes and Alkynes II: Addition Reactions Created by Professor William Tam & Dr. Phillis Chang Ch. 8 - 1 About The Authors These PowerPoint Lecture Slides were created and prepared by Professor William Tam and his wife, Dr. Phillis Chang. Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Guelph (Ontario, Canada) in 1998 and is currently a Full Professor and Associate Chair in the department. Professor Tam has received several awards in research and teaching, and according to Essential Science Indicators, he is currently ranked as the Top 1% most cited Chemists worldwide. He has published four books and over 80 scientific papers in top international journals such as J. Am. Chem. Soc., Angew. Chem., Org. Lett., and J. Org. Chem. Dr. Phillis Chang received her B.Sc. at New York University (USA) in 1994, her M.Sc. and Ph.D. in 1997 and 2001 at the University of Guelph (Canada). She lives in Guelph with her husband, William, and their son, Matthew. Ch. 8 - 2 1. Addition Reactions of Alkenes E C C + E Nu C C Nu Ch. 8 - 3 1A. How To Understand Additions to Alkenes This is an addition reaction: E–Nu added across the double bond E C C + E Nu C C Nu p-bond s-bond Bonds broken 2 s-bonds Bonds formed Ch. 8 - 4 Since p bonds are formed from the overlapping of p orbitals, p electron clouds are above and below the plane of the double bond C C p electron clouds Ch. 8 - 5 Electrophilic ● electron seeking ● C=C and C≡C p bonds are particularly susceptible to electrophilic reagents (electrophiles) Common electrophile ● H+, X+ (X = Cl, Br, I), Hg2+, etc. Ch. 8 - 6 In an electrophilic addition, the p electrons seek an electrophile, breaking the p bond, forming a s bond and leaving a positive charge on the vacant p orbital on the adjacent carbon. – Addition of B to form a s bond provides an addition product Ch. 8 - 7 C C E Nu E C C + Nu Nu E C C Ch. 8 - 8 2. Electrophilic Addition of Hydrogen Halides to Alkenes: Mechanism and Markovnikov’s Rule C Mechanism C E Nu A C C Nu Nu E C C Ch. 8 - 9 Mechanism ● Sometimes do not go through a “free carbocation”, may go via E C C Ch. 8 - 10 Markovnikov’s Rule ● For symmetrical substrates, no problem for regiochemistry H H C H C E Nu E H H C C H H same H as H E C C H H H Nu H E Nu C C H H same H as H Nu E C C H H H Ch. 8 - 11 Markovnikov’s Rule ● But for unsymmetrical substrates, two regioisomers are possible H H3C C H C E Nu E E CH3 H C C H H H or CH3 C C H H Nu Nu CH3 E Nu C C H H H different H from CH3 Nu E C C H H H Ch. 8 - 12 Markovnikov’s Rule ● In the electrophilic addition of an unsymmetrical electrophile across a double bond of an alkene, the more highly substituted and more stabilized carbocation is formed as the intermediate in preference to the less highly substituted and less stable one Ch. 8 - 13 Markovnikov’s Rule ● Thus E Nu E Nu E o o E NOT o Note: carbocation stability 3 > 2 > 1 Ch. 8 - 14 Addition of Hydrogen Halides ● Addition of HCl, HBr and HI across a C=C bond + ● H is the electrophile + H Br slow Br r.d.s fast Br Br NO Ch. 8 - 15 Ch. 8 - 16 2A. Theoretical Explanation of Markovnikov’s Rule H3C H C H C H H X step 1 (slow r.d.s.) H CH3 C C H H or CH3 C C H H H 2o carbocation H H 1o carbocation (more stable) (more stable) One way to state Markovnikov’s rule is to say that in the addition of HX to an alkene, the hydrogen atom adds to the carbon atom of the double bond that already has the greater number of hydrogen atoms Ch. 8 - 17 ☓ H H Br (1o cation) fast H (minor) Br Br slow (r.d.s.) H Br (2o cation) fast Step 1 H Step 2 Br (major) Ch. 8 - 18 Ch. 8 - 19 (1) Examples H Cl Cl H + H (95 (2) H Cl : 5) H Br Br + Br (98 H : 2) Ch. 8 - 20 2B. Modern Statement of Markovnikov’s Rule In the ionic addition of an unsymmetrical reagent to a double bond, the positive portion of the added reagent attaches itself to a carbon atom of the double bond so as to yield the more stable carbocation as an intermediate Ch. 8 - 21 Examples OH OH Cl (1) Cl OH Cl more stable (major) 3o cation Cl OH Cl OH less stable 1o cation (minor) Ch. 8 - 22 Examples Cl I (2) I Cl I Cl (major) more stable 3o cation Cl I less stable 1o cation Cl I (minor) Ch. 8 - 23 2C. Regioselective Reactions When a reaction that can potentially yield two or more constitutional isomers actually produces only one (or a predominance of one), the reaction is said to be regioselective H Cl Cl H + (major) H Cl (minor) regioisomers Regioselectivity: 95 : 5 Ch. 8 - 24 2D. An Exception to Markovnikov’s Rule H Br Br RO OR heat (anti-Markovnikov's product) H Via a radical mechanism (see Chapter 10) This anti-Markovnikov addition does not take place with HI, HCl, and HF, even when peroxides are present Ch. 8 - 25 3. Stereochemistry of the Ionic Addition to an Alkene H C Bu C H H X H Bu C H CH2 H C CH3 Bu (S)-2-Halohexane (50%) achiral trigonal planar carbocation Bu X attack from bottom racemate attack from top H X X H C CH3 X (R)-2-Halohexane (50%) Ch. 8 - 26 4. Addition of Sulfuric Acid to Alkenes OSO3H conc. H2SO4 cold O HO S H O H OSO3H O H more stable o 3 cation Addition of H–OSO3H across a C=C bond H less stable o 1 cation Ch. 8 - 27 4A. Alcohols from Alkyl Hydrogen Sulfates conc. H2SO4 cold OSO3H H H2O OH heat H The overall result of the addition of sulfuric acid to an alkene followed by hydrolysis is the Markovnikov addition of H– and –OH Ch. 8 - 28 5. Addition of Water to Alkenes: Acid-Catalyzed Hydration Overall process ● Addition of H–OH across a C=C bond + ● H is the electrophile ● Follow Markovnikov’s rule H2O dilute H3O+ (e.g. dilute H2SO4, H3PO4) OH H Ch. 8 - 29 5A. Mechanism H H O H2O H slow H H fast (step 2) (step 1) H more stable O H 3o cation fast (step 3) H2O H H O H H + OH Ch. 8 - 30 5B. Rearrangements Rearrangement can occur with certain carbocations H2O H H2SO4 1,2-alkyl shift NOT OH OH (major product) H2O Ch. 8 - 31 6. Alcohols from Alkenes through Oxymercuration–Demercuration: Markovnikov Addition Step 1: Oxymercuration C C Hg(OAc)2 THF-H2O C HO C HgOAc Step 2: Demercuration C HO C HgOAc NaBH4 OH C HO C HCh. 8 - 32 6A. Regioselectivity of Oxymercuration–Demercuration Oxymercuration–demercuration is also highly regioselective and follows Markovnikov’s rule HgOAc Hg(OAc)2 THF-H2O NaBH4 HO OH H HO Ch. 8 - 33 6B. Rearrangements Seldom Occur in Oxymercuration–Demercuration Recall: acid-catalyzed hydration of some alkenes leads to rearrangement products e.g. H2O H2SO4 OH H Ch. 8 - 34 H2O H2SO4 H 1,2-alkyl shift H O H H H2O H H2O OH H Ch. 8 - 35 Rearrangements of the carbon skeleton seldom occur in oxymercuration– demercuration no rearrangement OH 1. Hg(OAc)2, THF-H2O 2. NaBH4 via H OH Hg(OAc) Ch. 8 - 36 6C. Mechanism of Oxymercuration Does not undergo a “free carbocation” OAc Hg OAc HgOAc AcO + H2O HgOAc HO HgOAc H2O O H H Ch. 8 - 37 Stereochemistry ● Usually anti-addition Hg(OAc)2 H3C H THF-H2O H2O H3C Hg(OAc) OH H CH3 Hg(OAc) Ch. 8 - 38 Although attack by water on the bridged mercurinium ion leads to anti addition of the hydroxyl and mercury groups, the reaction that replaces mercury with hydrogen is not stereocontrolled (it likely involves radicals). This step scrambles the overall stereochemistry The net result of oxymercuration– demercuration is a mixture of syn and anti addition of –H and –OH to the alkene Ch. 8 - 39 Solvomercuration-Demercuration Hg(O2CCF3)2 OR THF-ROH Hg(O2CCF3) NaBH4 OH OR H Ch. 8 - 40 7. Alcohols from Alkenes through Hydroboration–Oxidation: Anti-Markovnikov Syn Hydration C C "BH 3" C C H BH2 Addition of H–BH2 across a C=C bond Ch. 8 - 41 BH3 exists as dimer B2H6 or complex with coordinative solvent H H H B B H H H H H B H O H (BH3-THF) H B Me S H Me (BH3-DMS) Ch. 8 - 42 syn addition 1. BH3-THF H OH CH3 H H3C H 2. H2O2, OH Anti-Markovnikov addition of “H” & “OH” Ch. 8 - 43 Compare with oxymercurationdemercuration Hg(OAc)2 H3C H THF-H2O OH H CH3 Hg(OAc) NaBH4 anti addition Markovnikov addition of “H” & “OH” OH H CH3 H Ch. 8 - 44 8. Hydroboration: Synthesis of Alkylboranes C C alkene +H B boron hydride hydroboration C C H B alkylborane Ch. 8 - 45 8A. Mechanism of Hydroboration H3C H H H H3C H + B H3C H H H H H H B H H3C H H H H H B H H p complex H H H B syn addition H of H and B H3C H H H four-atom concerted T.S. H H B H H H Ch. 8 - 46 (1) Other examples H BH2-THF + H BH2 H2B (99 (2) H BH2-THF : H 1) + H BH2 (98 H2B : H 2) Ch. 8 - 47 8B. Stereochemistry of Hydroboration Syn addition H3C H BH3-THF H BH2 CH3 H Ch. 8 - 48 9. Oxidation and Hydrolysis of Alkylboranes H H H H BH2 H B B always ends up on the least hindered carbon B B B H (trialkyl borane) H Ch. 8 - 49 Oxidation B O H2O2 O B O Ch. 8 - 50 ● Via R R R3B O OH R B O OH R R HO R O RO B R OR R O HO B OR R B O OR B OR O OH OH OR OR RO B OR Ch. 8 - 51 Hydrolysis O O B O NaOH H2O 3 OH + Na3BO3 Ch. 8 - 52 Overall synthetic process of hydroboration-oxidation-hydrolysis 1. BH3-THF 2. H2O2 3. NaOH, H2O H OH ● Overall: anti-Markovnikov addition of H–OH across a C=C bond ● Opposite regioisomers as oxymercuration-demercuration Ch. 8 - 53 anti-Markovnikov syn addition Example BH3-THF H3C H H OH CH3 H H BH2 CH3 H H2O2 OH This oxidation step occurs with retention of configuration Ch. 8 - 54 10. Summary of Alkene Hydration Methods Summary of Methods for Converting Alkene to Alcohol Reaction Regiochemistry Stereochemistry Occurrence of Rearrangements Acid-catalyzed hydration Markovnikov addition Not controlled Frequent Oxymercurationdemercuration Markovnikov addition Not controlled Seldom Hydroborationoxidation Anti-Markovnikov Stereospecific: addition syn addition of H – and –OH Seldom Ch. 8 - 55 Examples via H H H OH H H2O 1,2-hydride shift with rearrangement OH 1. Hg(OAc)2, THF-H2O H 2. NaBH4, OH Markovnikov addition of H2O without rearrangement H 1. BH3-THF 2. H2O2, OH anti-MarkovniOH kov, syn addition of H2O Ch. 8 - 56 11. Protonolysis of Alkylboranes R B alkylborane CH3CO2H heat R H + CH3CO2 B alkane Protonolysis of an alkylborane takes place with retention of configuration; hydrogen replaces boron where it stands in the alkylborane Overall stereochemistry of hydroboration– protonolysis: syn Ch. 8 - 57 e.g. 1. BH3-THF 2. CH3CO2D H H3C H3C H H D + enantiomer via H3C H H BH2 Ch. 8 - 58 12. Electrophilic Addition of Bromine and Chlorine to Alkenes Addition of X–X (X = Cl, Br) across a C=C bond C C Br2 CCl4 Br C C Br (vicinal dibromide) Ch. 8 - 59 Examples (1) Br Br2 Br + o 5 C Br (anti addition of Br2) Br (racemate) Cl (2) Ph Cl2 Ph 10oC 2 Ph (anti addition of Cl2) 1 Ph Cl Ph Ph Cl Cl same as (rotation of C1-C2 bond) Ch. 8 - 60 12A. Mechanism of Halogen Addition C C + Br Br C Br–Br bond becomes polarized when close to alkene C Br Br Br + Br Br (vincinal Dibromide) Br (bromonium) Ch. 8 - 61 Stereochemistry ● Anti addition Br H Br Br CCl4 H Br H Br Br H SN2 reaction enantiomer + (anti) Ch. 8 - 62 13. Stereospecific Reactions A reaction is stereospecific when a particular stereoisomeric form of the starting material reacts by a mechanism that gives a specific stereoisomeric form of the product Ch. 8 - 63 ● Reaction 1 H H3C CH3 H Br2 CCl4 CH3 C H Br H C H3C trans-2-Butene Br Br Br H H3C H CH3 (2R,3S)-2,3-Dibromobutane (a meso compound) ● Reaction 2 H H3C H CH3 cis-2-Butene H H Br2 Br CCl4 H H3C C CH3 + H3C C Br (2R,3R) Br C C Br H CH3 (2S,3S) (a pair of enantiomers) Ch. 8 - 64 Addition of bromine to cis-2-Butene (a) (a) H H3C C C H CH3 Br Br H3C Br (b) H H C C H Br C CH3 C H Br H3C (2R,3R)-2,3-Dibromobutane (chiral) CH3 Br bromonium ion (achiral) H H3C C Br C H Br CH3 (2S,3S)-2,3-Dibromobutane (chiral) (b) Ch. 8 - 65 Addition of bromine to trans-2-Butene (a) (a) H H 3C C C CH3 H Br Br H3C Br H (b) CH3 C H C CH3 C H Br H C Br H3C (R,S)-2,3-Dibromobutane (meso) Br bromonium ion (b) (achiral) H H3C C Br C CH3 Br H (R,S)-2,3-Dibromobutane (meso) Ch. 8 - 66 14. Halohydrin Formation C C X2 H2O OH C C X Addition of –OH and –X (X = Cl, Br) across a C=C bond + X is the electrophile Follow Markovnikov’s rule Ch. 8 - 67 Mechanism Br H3C H H2O Br H2O H3C Br H3C Br H OH H CH3 Br Ch. 8 - 68 Other variation ● If H2O is replaced by ROH, RÖH will be the nucleophile e.g. Br2 MeOH OMe Br Ch. 8 - 69 15. Divalent Carbon Compounds: Carbenes 15A. Structure and Reactions of Methylene CH2 N N Diazomethane heat or light CH2 + Methylene (a carbene) N N Nitrogen Ch. 8 - 70 CH2 N N H2C N N CH2 II I C C + CH2 N N III C C C Alkene Methylene H H Cyclopropane Ch. 8 - 71 15B. Reactions of Other Carbenes: Dihalocarbenes :CX2 (e.g. :CCl2) Generation by a-elimination of chloroform H Cl C Cl OtBu Cl t + CCl2 BuOH + Cl Ch. 8 - 72 Usually a syn (cis) addition across a C=C bond t H BuOK Cl CHCl3 H Cl (a cyclopropane) Ch. 8 - 73 Stereospecific reactions CCl2 CCl2 Cl Cl Cl Cl Ch. 8 - 74 15C. Carbenoids: The Simmons-Smith Cyclopropane Synthesis CH2I2 + Zn(Cu) (Zinc-copper couple) I ZnI C H2 (a carbenoid) Ch. 8 - 75 A stereospecific syn (cis) addition across a C=C bond CH2I2 Zn(Cu) (trans) (trans) CH2I2 Zn(Cu) (cis) (cis) Ch. 8 - 76 16. Oxidation of Alkenes: Syn 1,2-Dihydroxylation Overall: addition of 2 OH groups across a C=C bond C C OH OH ⊖ Reagents: dilute KMnO4 / OH / H2O / cold or OsO4, pyridine then NaHSO3, H2O Ch. 8 - 77 16A. Mechanism for Syn Dihydroxylation of Alkenes dil. KMnO4 OH, H2O cold C C O O Mn O C OH H2O O OsO4 pyridine C O O O Os NaHSO3 H2O O C OH OH + MnO2 C C C C C OH OH + Os Ch. 8 - 78 Both reagents give syn dihydroxylation dil. KMnO4 OH , H2O, cold H H H H or OsO4, pyridine then NaHSO3 OH OH (cis-diol) Ch. 8 - 79 Comparison of the two reagents ● KMnO4: usually lower yield and possibly side products due to overoxidation 1. KMnO4, + 2. H OH O + O (oxidative cleavage of C=C) OH ● OsO4: usually much higher yield but OsO4 is extremely toxic Ch. 8 - 80 17. Oxidative Cleavage of Alkenes 17A. Cleavage with Hot Basic Potassium Permanganate b a a b b KMnO4, OH , H2O 2 a O b H3O or a O a b O 2 b a OH Ch. 8 - 81 (1) Other examples 1. KMnO4, OH, H2O, heat 2. H3O O + O (2) 1. KMnO4, OH, H2O, heat 2. H3O C O O O OH Ch. 8 - 82 17B. Cleavage with Ozone R R' R" H 1. O3 2. Zn, AcOH or Me2S R R" O + O R' H Ch. 8 - 83 (1) Examples O 1. O3 2. Zn, AcOH O (2) O 1. O3 2. Me2S + H O Ch. 8 - 84 Mechanism C C C O O C O O C O C O O + C O O initial ozonide O C O O C O O Zn(OAc)2 + C O ozonide C O + O C Ch. 8 - 85 18. Electrophilic Addition of Bromine & Chlorine to Alkynes R R C C C C H H X2 X2 (excess) CH2Cl2 (X = Cl, Br, I) X H C H C X2 X (anti-addition) R R X X C C X X X X C C X X H H Ch. 8 - 86 19. Addition of Hydrogen Halides to Alkynes R H3C C C H H X (excess) R (X = Cl, Br, I) Regioselectivity ● Follow Markovnikov’s rule C C H HBr Br H C CH3 C H HBr X H C C X H H Br H CH3 C C H Br H gem-dibromide Ch. 8 - 87 Mechanism CH3 C C H H Br H CH3 C Br C H Br H C CH3 Br H CH3 C C Br H H Br H Br C CH3 C H H C H Br H Ch. 8 - 88 Anti-Markovnikov addition of hydrogen bromide to alkynes occurs when peroxides are present in the reaction mixture H Br peroxides Br H (E) and (Z) (74%) Ch. 8 - 89 20. Oxidative Cleavage of Alkynes R C C R' OR R Ph C C 1. O3 R' 2. HOAc 1. KMnO4, OH + 2. H3O RCO2H + R'CO2H RCO2H + R'CO2H Example C C CH3 1. O3 2. AcOH PhCO2H + CH3CO2H Ch. 8 - 90 21. How to Plan a Synthesis: Some Approaches & Examples In planning a synthesis we often have to consider four interrelated aspects: 1. Construction of the carbon skeleton 2. Functional group interconversions 3. Control of regiochemistry 4. Control of stereochemistry Ch. 8 - 91 21A. Retrosynthetic Analysis How to synthesize ? OH ● Retrosynthetic analysis OH (target molecule) (precursor) Ch. 8 - 92 ● Synthesis Markovnikov addition of H2O H+ H2O or OH 1. Hg(OAc)2, THF-H2O 2. NaBH4, OH Ch. 8 - 93 How to synthesize OH ? ● Retrosynthetic analysis OH (target molecule) (precursor) ● Synthesis 1. BH3-THF 2. H2O2, OH anti-Markovnikov addition of H2O OH Ch. 8 - 94 21B. Disconnections, Synthons, and Synthetic Equivalents One approach to retrosynthetic analysis is to consider a retrosynthetic step as a “disconnection” of one of the bonds In general, we call the fragments of a hypothetical retrosynthetic disconnection Synthons Ch. 8 - 95 Example How? Ph H Br Br CH3 Ph ● Retrosynthetic analysis (i) Br Ph Br CH3 Ph CH3 (gem-dibromide came from addition of HBr across a C≡C bond) Ch. 8 - 96 ● Retrosynthetic analysis synthons (ii) Ph CH3 Ph + CH3 disconnection H3C I + Ph Na synthetic equivalent Ch. 8 - 97 ● Synthesis Ph H NaNH2 liq. NH3 -30oC Ph Na H3C I (via an SN2 reaction) Br Ph Br CH3 HBr (excess) Ph CH3 Ch. 8 - 98 21C. Stereochemical Considerations How? H3C Br Br CH3 H3C CH3 Ch. 8 - 99 Retrosynthetic analysis ● The precursor of a vicinal dibromide is usually an alkene ● Bromination of alkenes are anti addition Br Br H3C Br H3C CH3 o (rotate 180 ) (anti addition of H2) H3C CH3 H3C CH3 Br (anti addition of Br2) CH3 Ch. 8 - 100 ● Synthesis H3C 1. Li, liq. NH3 CH3 H3C 2. NH4Cl CH3 (anti addition of H2) Br2/CCl4 Br H3C Br CH3 Br same as (anti addition of Br2) CH3 H3C Br Ch. 8 - 101 END OF CHAPTER 8 Ch. 8 - 102