Nomenclature - Clydebank High School
advertisement

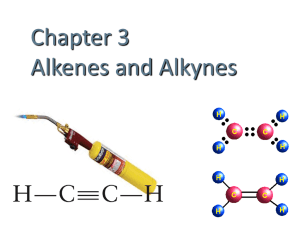
Homologous Series A group of Hydrocarbons with the same General Formula and similar chemical properties. Examples – Alkanes, Alkenes and Cycloalkanes. Alkanes Alkenes Cycloalkanes CnH2n+2 CnH2n CnH2n C-C single bonds At least 1 C=C doublebond Unsaturated Ring structure Saturated Saturated Naming hydrocarbons Nomenclature The prefix tells us how many carbons in molecule – e.g. meth = 1, eth = 2, prop=3. We number the carbon atoms, number 1 is always at the end closest to the main functional group. A functional group is a group of atoms with characteristic features e.g. A C=C bond is a functional group – showing the molecule is an alkene - unsaturated. Naming alkenes Example H H H H I I I I C = C – C - C– H This is but –1- ene I I I H H H H H H H I I I I H - C – C = C – C - H This is but –2- ene I I Alkynes General Formula C n H2n –2 They contain at least one C to C triple bond. They are unsaturated. They start with the usual prefix e.g. ethyne, propyne etc. They are named in the same way the alkenes are – in the main chain the carbons are numbered – number 1 is closest to the main functional group i.e. the triple bond. Example: but – 1 - yne Naming branched chain Alkanes 1. Select longest single straight chain – and name it. 2. Number C atoms in chain – number 1 is closet to branch. 3. Name branches: CH3 – methyl. C2H5 – ethyl, C3H7 – propyl etc. Example CH3 I CH3 – CH2 – CH2 – CH3 2 METHYL BUTANE 1 2 3 4 Branched Chain Alkenes 1. Select longest chain 2. Number Carbons – number 1 closest to C=C 3. Name any branches Example CH2=CH.CH3CH2CH2CH3 ( CH3 = branch) 1 2 3 4 5 2 methyl pent – 1 - ene Isomers Compounds with the same molecular formula but different structural formula. Alkenes and Cycloalkanes are isomers. Adding branches to chains increases the number of possible isomers. Example CH3CH2CH2CH2CH3 (C5H12) is an isomer of CH3CH.CH3CH2CH3 (C5H12) Alkanols ( alcohol) Another homologous series. They contain the functional group – OH This is called a Hydroxyl group They start with the usual prefix and end in ol. Example H Methanol CH3OH Ethanol CH3CH2OH I H-C–H I OH Naming Alkanols Carbon number 1 is the C closest to the Hydroxyl functional group. ( - OH ) Example CH3CH2CH2OH : propan – 1 ol CH3CH.OHCH3: Propan- 2 – ol. Primary Alcohols The functional group – OH is on a carbon which also has 2H atoms attached. Example Butan – 1- ol CH3 CH2CH2CH2OH Primary alcohols undergo oxidation reactions to form alkanals ( aldehydes) Secondary Alcohols The functional group – OH is on a carbon with which is also bonded to 1 H atom. Example Butan – 2- ol. CH3 CH2 CH(OH)CH3 Secondary alcohols oxidise to produce alkanones (Ketones) Tertiary Alcohols The functional group – OH is attached to a carbon with NO H atoms attached. Example 2 methyl propan – 2 – ol. C(CH3)3OH Tertiary alcohols can not be oxidised. Alkanals (aldehydes) Formed from the oxidation of primary alkanols. 2 H atoms are removed. Acidified potassium dichromate acts as an oxidising agent. Mix with alcohol – heat in water bath. Colour change orange – blue. ( Different smell) Alkanals are another example of a homologous series. They contain a carbonyl functional group. C=O ( at the end of a molecule) Example Ethanol will oxidise to produce Ethanal. CH3CH2OH —> CH3CHO H H H H I I I I H - C – C –OH —> H - C – C=O I I I H H H C=O is the carbonyl functional group Alkanones (Ketones) These are formed from the oxidation of secondary alkanols. The functional group – the carbonyl group – C=O is in the middle of the chain. I H atom is removed. Example Propan – 2 – ol will be oxidised to Propan – 2 –one. Example CH3CH(OH)CH3 —>CH3COCH3 H OH H H O H I I I I II I H – C - C - C – H —> H – C - C –C - H I I I I I H H H H H - C=O is the Carnbonyl functional group Alkanoic Acids Alkanoic acids are formed by the oxidation of alkanals (aldehydes) They are a subset of the Carboxylic acid group They contain a carboxyl functional group C=O ( COOH) I OH Alkanoic Acids Examples Ethanal will oxidise to give Ethanoic Acid CH3CHO —> CH3COOH H H H OH I I I I H - C – C =O —> H – C – C =O I I H H Alkanones can not be oxidised further. Esters Esters are formed from a condensation reaction between an alkanol and an alkanoic acid. Esterification. Esters have very distinctive smells Esters are insoluble in water. The first part of an ester name comes from the alkanol – the second part comes from the alkanoic acid. Example Methanol + Ethanoic Acid —> Methyl ethanoate + Water Ester examples Ethanol + Methanoic acid —> Ethyl methanoate H H OH + Water I I I H – C – C – OH + O=C I I I H H H Ester formed H H O I I II H - C – C – O – C – H + H2O I I H H O II This is the ester link -O–C- More Examples Ethanol+ Butanoic Acid —> Ethyl butanoate + Water Propanoic Acid + Methanol —> Methyl propanoate + Water Uses of Esters Esters can be used as non polar solvents. Example – Ethyl ethanoate is nail polish remover! They are used to add flavour and taste to many substances. When an ester is made there are 2 very noticeable changes: 1. The smell 2. It is immiscible with water – we can see the separate layers. Esters Shortened Structural Formula Ethyl methanoate H H O I I II H – C –C - O -C – H I I H H CH3CH2OCOH Methyl ethanoate H O H I II I H–C–O–C-C-H I I H H CH3OCOCH3 More! H O H H H I II I I I H – C – C –O – C – C – C – H I I I I H H H H CH3COOCH2CH2CH3 Propyl ethanoate Hydrolysis of Esters This is the opposite of a condensation reaction. We are splitting the ester - back into the alkanol and alkanoic acid. We must add back the water which is removed in the condensation reaction. This is not very successful with water alone so we add a dilute acid to catalyse it e.g. HCl or H2SO4. (Or an alkali.) They provide H+ ions to catalyse the reaction. It is a reversible reaction ( PPA – 2) Aromatic Hydrocarbons They are a subset of hydrocarbons. Benzene is the simplest aromatic compound – C6 H6 Each carbon has 3 ½ filled electron clouds which bond with the nearest atom – delocalised electrons. When we replace one of the H atoms with another group we have a phenyl group C6 H5 Examples C6H5 – CH3 = methyl benzene (Toluene) C6H5 – OH = Phenol C6H5 – COOH = Benzoic Acid C6H5 – NH2 = Phenyl amine. Uses of Benzene It is an important feedstock. It used to produce: Cylco hexane Ethyl benzene Phenol Alkyl benzenes Note : Although benzene contains delocalised electrons – they are contained within the ring – benzene does not conduct electricity. Reactions of Carbon Compounds Revision from SG Cracking – using heat/catalyst to break heavier fractions into smaller more useful ones. Addition reactions – adding atoms to unsaturated compounds e.g. alkenes. Example – decolourisation of Br2( aq) instantly. Addition reactions - Alkynes Alkynes can undergo a 2 stage addition reaction to become saturated. Example Ethyne + Hydrogen —> Ethene (Unsaturated) Ethene + Hydrogen —> Ethane ( Saturated) Addition reactions with Halides Ethyne + Bromine —> Bromoethene ( unsaturated) Bromoethene + Bromine —>Bromoethane ( saturated) We can use Bromine solution as a test for unsaturated compounds on alkenes and alkynes. Ethanol Ethanol can be produced in 2 ways: 1. Fermentation of glucose 2. Addition of water to alkenes using a catalyst – catalytic hydration. Example H H H H I I I I C = C + H2O —> H – C – C - OH I I I I H H H H Dehydration of alcohols We can convert Ethanol to Ethene by dehydration. We soak mineral wool in the alcohol and heat in presence of a catalyst. Aluminium oxide can act as a catalyst in the lab. Examples Butan – 1 – ol will become But – 1 - ene. Butan – 2 – ol can produce both But – 1 - ene and But – 2 – ene. % Yield The yield is the quantity of the product obtained. % yield is when we calculate the actual yield as a % using the theoretical yield. Example 5g of Methanol reacts with excess Ethanoic acid to produce 9.6g of methyl ethanoate. Steps 1. 2. 3. 4. 5. 6. Balanced equation CH3OH + CH3COOH <=> CH3COOCH3+ H2O Number of moles I mol Methanol----> 1 mol Methyl ethanoate Put in mass - Theretical 32g ----------------> 74g Actual mass 5g -----------------> 74/32 x 5 11.56g = Theoretical Yield Actual Yield = 9.6g % Yield = Actual/Theoretical x 100 = 9.6/11.56 x 100 = 83%