Electron_structure
advertisement

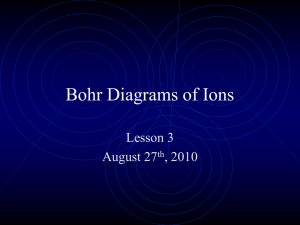
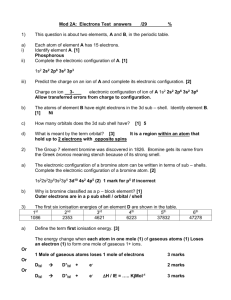
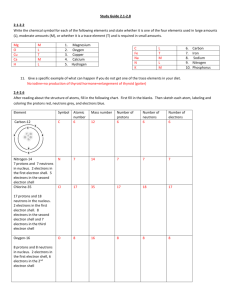
Lesson 2 Arranging the electrons and patterns in electron configuration The 2, 8, 8 rule For the GCSE exam you will be required to arrange the electrons for the first 20 elements in the Periodic Table. The arrangement of electrons in an atom is called the Electron Configuration. Shell or orbit (starting with the one closest to the nucleus) 1st 2nd 3rd 4th Maximum number of electrons that can be placed in the shell or orbit 2 8 8 Any left over Drawing an electron configuration Step 1. Draw a blob for the nucleus. Na Step 2. Draw the first shell. Add 2 electrons. Step 3. Draw the second shell. Add 8 electrons. Step 4. Draw the third shell. Add the last remaining electron. K K 2, 8, 8, 1 Group Number of electrons in outer shell 1 2 3 4 5 6 7 0 or 8 1 2 3 4 5 6 7 full When is an atom not an atom? Atoms react with other atoms by losing or gaining electrons. An atom that has lost or gained electrons is called and ION. An atom that has lost electrons (lost negative charges) is called a positive ion and an atom that has gained electrons (gained negative charges) is called a negative ion. In other words an ion is a charged particle. How many electrons are lost or gained depends upon in which Group the element belongs. The aim is to achieve a full outer shell of electrons (in most cases this is 8 electrons). Group Number of electrons in outer shell Number of electrons lost or gained Charge of ion 1 2 3 4 5 6 7 0 or 8 1 2 3 4 5 6 7 full Group 0 or 8 He 2 Ne 2, 8 Ar 2, 8, 8 Kr 2, 8, 18, 8 Xe 2, 8, 18, 18, 8 Rn 2, 8, 18, 32, 18, 8 The atoms of these elements already have a full outer shell. This is why these elements don’t react. Hence they are called the Inert or Noble gases. Note the 2, 8, 8 rule only works for the first 20 elements