Naming Covalent Compounds Worksheet
advertisement

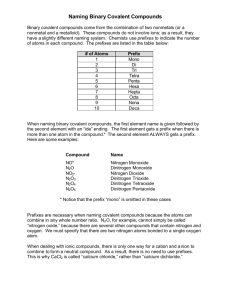
Name ____________________________________________________Date ______________________ Period_______ Naming Covalent Compounds Worksheet Chemistry; Coleman Covalent compounds- consist of nonmetals only. Naming Rules: 1. The first element is named first, using the element’s name off the periodic table 2. For the second element, take its root and add ending –ide 3. Add prefixes which will show how many atoms of each element are here in a compound 4. “Mono” is not used to name the first element. Prefixes: 1: mono 2: di 3: tri 4: tetra 5: penta 6: hexa 7:hepta 8: octa 9: nona 10: deca Note: when the addition of the Greek prefix places two vowels adjacent to one another, the "a" (or the "o") at the end of the Greek prefix is usually dropped; e.g., "nonaoxide" would be written as "nonoxide", and "monooxide" would be written as "monoxide". The "i" at the end of the prefixes "di-" and "tri-" are never dropped. Examples: 1. H2O2 : dihydrogen dioxide 2. P2S3 : diphosphorous trisulfide 3. CO2: carbon dioxide Write the formulas for the following covalent compounds: 1) antimony tribromide __________________________________ 2) hexaboron monosilicide __________________________________ 3) chlorine dioxide __________________________________ 4) trihydrogen monophosphide __________________________________ 5) iodine pentafluoride __________________________________ 6) dinitrogen trioxide __________________________________ 7) carbon monoxide __________________________________ 8) phosphorus triiodide __________________________________ Write the names for the following covalent compounds: 9) P4S5__________________________________ 10) O2 __________________________________ 11) SeF6 __________________________________ 12) Si2Br6 __________________________________ 13) SCl4 __________________________________ 14) CH4 __________________________________ 15) B2Si __________________________________ 16) NF3 __________________________________ Name the following chemical compounds. Some are Ionic and some are covalent. Remember, you do not use the prefixes when naming Ionic compounds. Be careful… 1) NaBr ______________________________________________ 2) Ca(C2H3O2)2 ______________________________________________ 3) P2O5 ______________________________________________ 4) Ti(SO4)2 ______________________________________________ 5) FePO4 ______________________________________________ 6) K3N ______________________________________________ 7) SO2 ______________________________________________ 8) CuOH ______________________________________________ 9) Zn(NO2)2 ______________________________________________ 10) V2S3 ______________________________________________