How to Name Ionic and Covalent Compounds-easy for Physical Science
advertisement
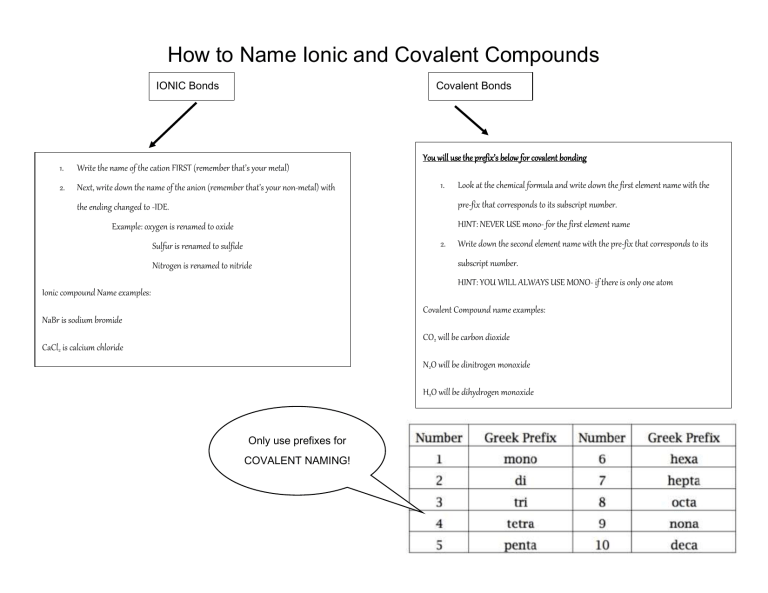
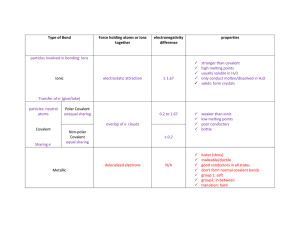
How to Name Ionic and Covalent Compounds IONIC Bonds Covalent Bonds 1. Write the name of the cation FIRST (remember that’s your metal) 2. Next, write down the name of the anion (remember that’s your non-metal) with You will use the prefix’s below for covalent bonding 1. Look at the chemical formula and write down the first element name with the pre-fix that corresponds to its subscript number. the ending changed to -IDE. HINT: NEVER USE mono- for the first element name Example: oxygen is renamed to oxide 2. Sulfur is renamed to sulfide Nitrogen is renamed to nitride Write down the second element name with the pre-fix that corresponds to its subscript number. HINT: YOU WILL ALWAYS USE MONO- if there is only one atom Ionic compound Name examples: Covalent Compound name examples: NaBr is sodium bromide CO2 will be carbon dioxide CaCl2 is calcium chloride N2O will be dinitrogen monoxide H2O will be dihydrogen monoxide Only use prefixes for COVALENT NAMING!