Covalent Bond Practice
advertisement

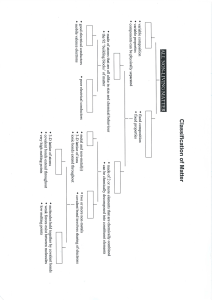
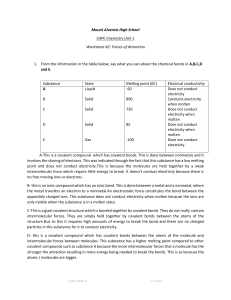
Name_______________________________ Date_______________ Hour__________ Covalent Bonding Practice 1) Explain the difference in how ionic and covalent bonds are held together. 2) HCl is a polar molecule. Label each element with its partial charge and example why the charge occurs. 3) Label the following bonds with single, double or triple and label the total number of electrons shared between the atoms. (Hint: Draw the electron dot diagrams). a) CH4 b) NP c) NCl3 4) What is a diatomic element and list all 7. 5) What are the properties of covalent bonds? 6) Covalent bonds are made of what types of elements? d) O2 7) Write the formulas or compound names for the following: SO3 ___________________________ CO _____________________________ CS2 _____________________________ CCl4 ____________________________ N2O _____________________________ As2S3 ______________________________ NI3 _____________________________ Phosphorus trichloride ___________________________________ Diphosphorus pentoxide ___________________________________________________ Dinitrogen pentoxide _____________________________________ Dinitrogen pentasulfide __________________________________ Oxygen difluoride _________________________________________ Tetraphosphorus decaoxide _______________________________ Silicon tetrabromide ______________________________________ Sulfur dioxide _____________________________________________ Bromine monochloride ____________________________________ Diarsenic pentasulfide _____________________________________