Worksheet for Chapter 9 PSC1515
advertisement

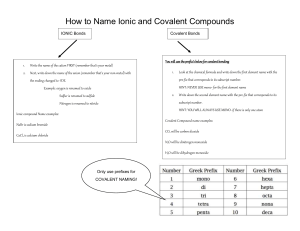
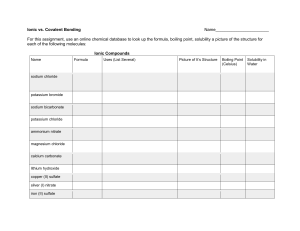
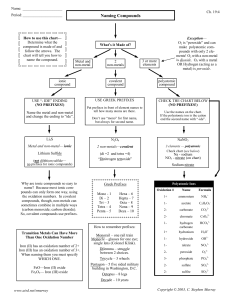
Worksheet for Chapter 9 PSC1515 1. Identify the following elements as monoatomic (m) or diatomic (d): a. nitrogen____________ b. carbon____________ c. potassium___________ d. fluorine______________ 2. Identify the following elements as solids (s) , liquids (l) , or gases (g) at room temperature: a. sodium_______________ b. argon______________ b. bromine_______________ d. mercury______________ e. sulfur________________ e. fluorine______________ 3. Identify whether the following are composed of atoms (A) , molecules (M), or formula units (FU): a. CaCl2 ____________ b. Cl2 __________ c. Fe____________ d. CO2 ____________ e. Na 2 SO4____________ 4. What is the charge that each of the following elements acquires when it forms ions? a. Na____________ b. C ___________ c. I ______________ d. Ba____________ e. S ____________ 5. How many valence electrons does each of the following elements have? a. neon____________ b. phosphorous_____________ b. calcium__________ d. hydrogen__________ e. bromine________ 6. What is the electronic configuration for each of the following elements? a. Mg _____________ b. C_____________ c. Ar_____________ d. K______________ 7. What is the formula for the compound formed from each of the following pairs of elements? a. K and O _______________ b. Al and S _____________ b. Mg and N _______________ d. Ca and Se ______________ 8. What is the formula formed when the following element and/or polyatomic ion combine? a. lithium and sulfate__________ b. ammonium and phosphate_________ c. beryllium and carbonate_____________ d. Pb4+ and oxalate_____________ 9. Name the following: a. MgCO3 __________________ b. Ca (OH)2___________________ c. Ga (NO3)3 ____________________ d. (NH4)3N ____________________ e. K3P _________________ f. Cu2I ________________ g. Cl2O7_______________ h. NO _________________ 10. Provide the formulas for the following: a. iron (III) oxide _______________ b. sodium bromide_______________ c. cobalt (II) phosphate______________ d. aluminum carbide ________________ e. dinitrogen monoxide ________________ 11. Which of the following are electrolytes (E) or nonelectrolytes (NE)? a. solid ionic compounds ____________ b. covalent compounds dissolved in water _____________ c. ionic compounds dissolved in water _____________ d. solid covalent compounds _______________ e. metals ________________ 12. Which of the following elements is more electronegative based on its position in the periodic table? (Do not look at EN values for this) a. F or Na __________ b. Cl or I ___________ c. C or N _____________ d. P or N _______________ 13. Check the EN values for the elements in the following bonds and whether the bond is polar covalent (PC), nonpolar covalent (NC), or ionic (I): a. HBr__________ b. KBr _____________ c. NO _____________ e. O2 ______________ f. CH4 ___________ Answers: 1. d, m, m, d 2. s, g, l, l, s, g 3. FU, M, A, M, FU 4. +1, -4, -1, +2, -2 5. 8, 5, 2, 1, 7 6. 2-8-2, 2-4, 2-8-8, 2-8-8-1 7. K2O, Al2S3, Mg3N2, CaSe 8. Li2SO4, (NH4)3PO4, BeCO3, Pb(C2O4)2 9. magnesium carbonate, calcium hydroxide, gallium nitrate, ammonium nitride, potassium phosphide, copper (II) iodide, dichlorine heptoxide, nitrogen monoxide. 10. Fe2O3, NaBr, Ca3(PO4)2, Al4C3, N2O 11. NE, NE, E, NE, E 12. F, Cl, N, N 13. PC, I, PC, NC, NC