Ch 14 power point
advertisement

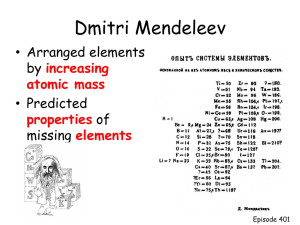
Chapter 14 Periodic Trends CP Chemistry Fall 2013 Chapter 14: Chemical Periodicity • 14.1 Objectives – Explain why you can infer the properties of an element based on those of other elements in the periodic table. – Use electron configuration to classify the elements as • • • • Noble gases Representative elements Transition metals Inner transition metals The Periodic Table • Russian chemist Dmitri Mendeleev, in 1871, created a successful organizational scheme for the periodic table. • The periodic table is useful for understanding and predicting the properties of the elements. – If you know the chemical and physical properties of one element of a group (vertical column) you can forecast the properties of the other elements in that column too. • The electron plays the most significant role (more than protons or neutrons) in determining the physical and chemical properties of an element. – This is because it is on the “outside” of the atom • Therefore there is a relationship between the electron configuration and the placement of elements in the table. Periodic_table_of_elements_showing_electron_shells.png See how you add one electron to the outer shell as you move from left to right? Shell 2 contains the 2s and 2p orbitals for a total of 8 electrons. Which element has 8 electrons in the 2nd shell? See how you add a new outer shell every time you start a new row of the periodic table? Period 1 has 1 shell Period 2 has 2 shells Period 3 has 3 shells and so forth… Then if you compare Be to Li, see how it has one more electron in the outer shell? Same thing if you compare Potassium and Calcium. See how one electron got added to the outer shell. But what happens with Scandium? Why aren’t there 3 e- in the outer shell? There is an extra one but where is it and why?? Noble Gases • These are elements where the outermost s and p sublevels are filled up (2 electrons in each “square” (orbital), all squares in Aufbau diagram are full. • The noble gases are called Group 0. • These elements are also called inert gases because they are very unreactive with other elements. – Their outer sublevels are “full” so they don’t want to gain or lose any more electrons. Noble gases (continued) 2 He 2 8 Ne 2 8 8 Ar 2 8 18 8 Kr • Look at the periodic table on page 393. Note the top right hand corner shows the electrons in each energy level. • He 1s2 (total 2) • Ne 1s22s22px22py22pz2 or 1s2 2s22p6 (total 10) • Ar 1s2 2s22p6 3s23p6 (total 18) • Kr 1s2 2s22p6 3s23p63d10 4s24p6 (total 36) Representative Elements • For these elements, the outermost s or p level is partially filled. • The representative elements are usually called Group A elements. – Sometimes the noble gases are also included in Group A. • Group 1A (1st column on far left) elements are called alkali metals • Group 2A (2nd column) elements are called alkaline earth metals. • Group 7A (2nd to the right column) elements are called halogens. • For any representative element, the number (2A, 7A) equals the number of electrons in the outermost energy level. • Example Group 1A: One electron in outer sublevel (n=2,3,4) • Li n=2 1s2 2s1 • Na n=3 1s2 2s2 2p6 3s1 • K n=4 1s2 2s2 2p6 3s2 3p6 4s1 Representative Elements (cont.) • Let’s try Group 4A. It should have 4 e- in outer sublevel: – C 1s2 2s2 2p2 (2 e- in 2s, 2 e- in 2p, total=4) – Si 1s2 2s2 2p6 3s2 3p2 (4 e- in sublevel 3) – Ge 1s2 2s2 2p6 3s2 3p6 4s2 4p2 (4 e- in sublevel 4) • How many e- in outer sublevel/shell for – – – – Mg ? P? B? I ? The transition metals • These are metallic elements in which the outermost s sublevel and nearby d sublevel contains electrons. • The transition elements, called the Group B elements, are characterized by the addition of electrons to the d-orbitals. • Example: Sc in column 3B – 1s2 2s2 2p6 3s2 3p6 3d1 4s2 • Did you notice that the d level you are filling is one less than the last s level you filled? (3 vs. 4) The inner transition metals • These are metallic elements in which the outermost s sublevel and the nearby f sublevel generally contain electrons. • The inner transition metals are characterized by the filling of the f-sublevels. • When you are filling f-sublevels, you will be working with a number 2 less than the last slevel filled. For example, when you finish with 6s2 then you will be filling the 4f level • • • • Note that the d-orbitals are associated with the transition metals. The f-orbitals are associated with the inner transition metals. Note how the d-levels are one less than the matching s-levels. The f-levels are two less than the matching s-levels. • If you consider electron configurations and the position of the elements in the periodic table, a new pattern can be seen. The periodic table can be divided into blocks that correspond to which sublevels are filled with electrons. Outer shell electrons • s-block – how many outer electrons? – Is He included? – How many electrons does it have? • p-block – what groups does it include? – Does it include He? Electron configurations • Electron configurations can be found by reading the periodic table like you read a book, from top to bottom, left to right. • Each period (line you are reading) corresponds to one principal energy level (n=1,2,3) • You can find the number of e- in each sublevel by counting from left to right. • For transition elements, e- are added to a d sublevel with principal energy level one less than the period number. – Example: if you are in row 4, you will be adding e- to level 3d. • For inner transition elements, it’s the same except for f sublevels it will be two less than the principal energy level. – Example: If you are in row 6, adding e- to level 4f Sample problem 14-1 • Write the electron configuration for N and Ni. a. N has 7 protons (atomic number) and therefore 7 e-. It is in row 2 so it’s principal energy level = 2. Start filling until you get to 7 total: 1s2 2s2 2p3 N is the 3rd element over in the p-block so it makes sense that it should have 2p3 b. Ni has 28 protons and 28 e-. Its in row 4, so n=4 1s2 2s2 2p6 3s2 3p6 3d8 4s2 total superscripts = 28 (recall you are filling d-level 3 after s-level 4, so dlevel 3 isn’t full yet…) Section 14.2 – Periodic Trends • Objectives – – Interpret group trends in • • • • Atomic radii Ionic radii Ionization energies Electronegativity – Interpret period trends in the same four items listed above. Atomic radii • To measure atomic size (atomic radii), x-ray diffraction can be used to measure the distance between nuclei of a diatomic molecule like H2, Cl2 or O2. • 1 pm = 1 picometer = 1x10-12m Atomic radii What trends in atomic radii do you see ? • Look at the top right corner for size in pm (picometers). • Look at size of atom drawn in top left corner. • Ignore ionic radii (bottom of cells) for now. Group Trends – Atomic Radius • Atomic size generally increases as you move down a group in the periodic table. • As you move down, there are more e- being added to successively higher energy levels, so that the outer orbital is further out as you move downwards. • The nuclear charge (# protons) is also increasing at the same rate. The inner orbitals filled with e- have a shielding effect. • You might expect that the + charge in the nucleus would pull e- towards the center and shrink the size of the atom, but that isn’t what happens. • The enlarging effect of the greater distance of the outer electrons overcomes the shrinking effect caused by the increased + charge in the nucleus. Atomic vs. Ionic radii • Note how the atomic radius (grey bars) increase as you go down the group (from atomic radius: Li < Na < K < Rb < Cs • Now look at the blue bars. These are positive ions. That means that one outer shell electron has been stolen from the atom. Why do you think that makes the atomic radius smaller (why are the blue bars shorter than the grey bars)? • Look at the bottom chart for the halogens. If you now add an e-, the ionic radius gets larger, why? Periodic Trends You may see the attraction between oppositely charged particles, such as an electron and a proton, called Coulombic attraction in your homework. • Atomic size generally decreases as you move from left to right across a period. • As you go across a period, the principal energy level is constant. As you go across, one proton and one e- are being added per square. • The effect of the added protons to the nucleus is to attract the electrons closer to the nucleus, shrinking atomic size. • If you plot an atomic radius (y-axis) vs. atomic number (x-axis) you get the graph here. Quick Quiz 1 – Why is atomic radius going down from Li to Ne? • • • • • • • • • Li Be B C N O F Ne Na 1s2 2s1 1s2 2s2 1s2 2s2 2p1 1s2 2s2 2p2 1s2 2s2 2p3 1s2 2s2 2p4 1s2 2s2 2p5 1s2 2s2 2p6 1s2 2s2 2p6 3s1 1 outer e2 outer e3 outer e4 outer e5 outer e6 outer e7 outer e8 outer e-, full p-shell Why is Na radius larger?? • Quick quiz #2 – • Can anyone explain why the size jumps up with Na? • Neon had 10 protons, Na has 11 protons, wouldn’t the addition of one more proton make the atom smaller due to attraction from the nucleus? • It is bigger because now that all the 2p orbitals are full, the last e- went in the 3s shell (n=3 principal number), which is further out from the nucleus, making the atom larger. • QQ #3: Why is period 4’s downward trend more gradual than period 2’s trend? • There is a shielding effect that occurs for the higher electron counts. The nucleus does have a lot of + charge, but the numerous inner e- interfere and shield the effect of the nucleus pulling in the outer e- as much. Trends in Atomic Radii Summary Memorize this page for the test! Trends in Ionization Energy • When a neutral atom gains or loses an e-, it becomes an ion. (Note it doesn’t lose/gain any protons or neutrons). • The + nucleus exerts an attractive force on all its e-. It doesn’t want to lose any e-, so to remove an e- requires energy to overcome this force. This amount of energy (different for every element) is called the ionization energy. • Na (g) Na+(g) + e• The energy required to remove the first e- is called the first ionization energy. If a second e- is removed, the energy required to do that is the second ionization energy. • Mg Mg2+ + 2e- Look at oxygen • What are its 1st, 2nd and 3rd ionization energies? 1314, 3391, 5301 kJ/mol • What do you notice about the trend? Each electron gets harder to remove. Look at Na • Why is there a dashed line between the 1st and 2nd ionization energies? Na 1s2 2s2 2p6 3s1 1st ioniz. energy: 495 2nd ioniz. energy: 4565 First e- takes partially filled 3s1 e-, 2nd e- breaks into full p-shell, doesn’t want to give up that e-. Look at Li >> Ne: • What trend do you notice in 1st ionization energy? • Ionization energy increases • Can you hypothesize why that might be? • From B to Ne, the closer the pshell is to being full, the more it wants to stay full and the more difficult it is to steal an e-. • Why is the first ionization energy for B lower than that for Be? That doesn’t follow the trend. • Be has a full 2s2 shell, doesn’t want to have that e- stolen. B has only 1 e- in p-shell, doesn’t mind losing it. Why do you think the first ionization energy decreases as you move down a group? For example: Li 520 kJ/mol Na 495 kJ/mol K 419 kJ/mol This is because the size of the atoms increases as you go down. As there are more e-, they fill orbitals/shells that are further out from the nucleus, which means the attractive force from the nucleus is less because they are farther away. Therefore they are easier to steal. Trends in Ionization Energy Notice how those noble gases (He, Ne, Ar…) are not willing to give up their fullshell electron which means they have a high first ionization energy. For the representative elements, first ionization energy increases going from left to right. As you move across, it adds a p+ and an e- with each square. A greater charged nucleus = a greater attraction = less willing to give up an e-. Ionization Energy Trends Memorize this chart for the test! QQ: Which group 6A element has the highest first ionization energy? Which element in period 2 has the highest ionization energy? Naming Ions • Before we start discussing the trends in ionic radii, let’s first define a few things: • Cation – a positive ion. That means that an electron has been stolen from the neutral atom and it has 1 more proton than electron. • Anion – a negative ion. It has a negative charge because it has stolen an electron from some other atom, meaning it has one more e- than it does protons, giving it a negative charge. • Refer to pages 135-136 in your book for this content (It is in Ch. 6, which we haven’t read yet.) Naming Cations – positive ions • Let’s say sodium, Na, has 11 protons and only 10 electrons (one has been stolen). How do we write that ion? Na+ called “Sodium ion” • What if we had Ca, with 20 protons and only 18 electrons (the two outer shell e- have been stolen). How do we write that ion? Ca2+ and we call that “Calcium ion” Naming Metal Cations • This actually comes from Ch. 6 page 144, but let’s have a preview now: • Cu+ ion is called a Copper(I) ion. • Cu2+ ion is called a Copper(II) ion. • Sn2+ ion is called a Tin(II) ion. You say “Tin two ion” • Sn4+ ion is called a Tin(IV) ion. You say “Tin four ion”, etc. • Mn2+ ion is called what? • Mn3+ ion is called what? • Note that many metals can have more than one ionic charge (although a particular one may be most common). • Note that you don’t put any space between the name and the parenthesis that follow it. • Need to know this for this test! Naming Anions – negative ions • Atoms of nonmetallic elements tend to form anions because they want to add e- to fill their shell until it looks like a noble gas. • Cl- has 17 protons and 18 e-, one extra that it has stolen. This is called a “chloride anion.” • Note the –ide ending on anions! • O2- is the oxide anion that you get when oxygen, with 8 protons, gains/steals two eand then has 10 e-. The superscript is telling you that it has two too many electrons relative to its neutral form. Why does it change size so much when it becomes an ion? You are about to find out. Trends in Ionic Radii/Size • The atoms of metallic elements have low ionization energies, which means they form positive ions easily. • That means they don’t hold onto their outer every strongly. That’s why metals conduct electricity, there is a “sea of electrons” surrounding the metal nuclei and those free ebecome the electric current when they freely move. • By contrast, nonmetallic elements readily form negative ions (they steal e-). • Can you explain why that Li atom got smaller after one electron got stolen from it? • Li is 1s2 2s1 . If it’s outer electron gets stolen, then it’s back to the 1s orbital, which is closer to the nucleus. The same thing occurs with Na and K, who each have only one outer e-. • What about Aluminum when 3 e- get stolen? • Al has three p electrons in the outer 2p shell. When all three are stolen, it is also back to it’s 1s orbital, which is smaller. • Now what about the halogen atoms, F, Cl and Br … Why does their size get much larger when one electron is added to them? • Let’s start with Fluorine 1s2 2s2 2p5 • This has 7 outer shell electrons (needs 8 to be full), so it is not larger because the last e- goes into 3s1, it doesn’t – it goes 2p6. So why is the – (negative) ion greater in size? • Now you have one more e- than you do protons, so the force per e- is now weaker than it is when the number matches, so it expands. Group Trends - Ions • Positive ions (cations) are always smaller than the neutral atom they start out with – why? • The number of protons is still the same, so the force per e- is stronger and it pulls it inward. • In contrast, negative ions (anions) are always larger than the neutral atom because now the same proton charge has 1 extra e- to attract, which makes the average force per e- less. • See graphic on next page… Now look again at ionic radii Periodic Trend for Ionic Size/Radii MEMORIZE THIS FOR TEST !! >>> • Going from L to R across a row, there’s a gradual decrease in the size of positive ions. • Then, starting with group 5A (Nitrogen), the negative ions, which are much larger, gradually decrease in size as you move from L to R also. – N3- O2- F- : trend makes sense, each one has fewer extra electrons so the effect is smaller. Last but not least, trends in electronegativity • The electronegativity of an atom is the tendency of an atom to attract (steal) e- when it is chemically combined with another element. • Relative electronegativity values are expressed on the Pauling electronegativity scale. • Highest: F 4.0 Lowest: Cs 0.7 • What trends do you see going across a period L to R? Why? • Down a group? Why? Electronegativity • Metallic elements at far left have low electronegativity. – Remember earlier when we said the e- for metals are loosely associated with the nucleus like a sea of electrons? • Nonmetallic elements at far right have high electronegativity. – Fluorine is the highest at 4.0 – its shell lacks just 1 e- to have a full 2p6 so it really “wants” that e-. Since F is so likely to steal an e-, it naturally forms F- ions. – Cesium, with electronegativity of 0.7 only has one outer shell e-, and it easily gives it up to form Cs+ ions. Use a similar argument for Na+ ions. • Electronegativity values are used to predict the type of bonding that will occur between 2 atoms. Na-Cl becomes Na+Cl- ionic bonding, while a C-H bond with electronegativity values of 2.1 and 2.5 forms a covalent bond where the e- are shared rather than “stolen”. Summary Chart – Need to memorize QQ#1 : Explain why atomic radius goes down as you go L to R. Shielding is constant as you go L to R (same principal quantum number) but more protons mean greater attraction to pull e- in that shell inwards. Summary Chart – Need to memorize QQ#2 : Explain why ionization energy goes up as you go from L to R. Group 1 – willing to give up its one outer electron easily. Group 0 – full outer shell, doesn’t want to lose any e- Summary Chart – Need to memorize QQ#3 : Explain why electronegativity increases as you go from L to R (up to Group 7). Group 1 – willing to give up its one outer electron easily. Group 7 – Badly wants to steal an e- to complete its principal energy level’s outer shell Summary Chart – Need to memorize QQ#4 : Why is nuclear charge increasing from L to R? Nuclear charge is increasing because there is one more proton each time you move one square to the right. Summary Chart – Need to memorize QQ#5 : Why does atomic radius go up as you go down a group? Protons are being added to the nucleus, and therefore e- are filling shells farther out from the nucleus, increasing the size of the atom. Summary Chart – Need to memorize QQ#6 : Why does ionization energy go down as you go down a group? Because outer e- are further from the nucleus and so there is less attractive force from the nucleus out there, meaning it is easier to steal an e-. LAST SLIDE !!