subshell recap ions and metalic bonding
advertisement

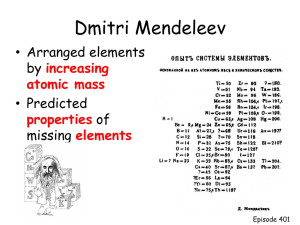
To begin with... A recap on subshells Electron subshell recap • Question: Write out the full electronic subshell for the element: Si • 1s2 2s2 2p6 3s2 3p2 Electron subshell recap • Question: Write out the full electronic subshell for the element: Si • 1s2 2s2 2p6 3s2 3p2 Electron subshell recap • Question: Write out the full electronic subshell for the element: Si • 1s2 2s2 2p6 3s2 3p2 Electron subshell recap • Question: Write out the full electronic subshell for the element: Si • 1s2 2s2 2p6 3s2 3p2 Si Electron subshell recap 1s2 • Question: Write out the full electronic subshell for the element: Si • 1s2 2s2 2p6 3s2 3p2 Si 1s2 Electron subshell recap 1s2 2 • Question: Write out the full electronic 2s subshell for the element: Si • 1s2 2s2 2p6 3s2 3p2 Si 1s2 2s2 Electron subshell recap 1s2 2 • Question: Write out the full electronic2p6 2s subshell for the element: Si • 1s2 2s2 2p6 3s2 3p2 Si 1s2 2s2 2p6 Electron subshell recap 1s2 2 • Question: Write out the full electronic2p6 2s 2 for the element: Si 3ssubshell • 1s2 2s2 2p6 3s2 3p2 222s222p663s22 SiSi 1s 1s 2s 2p 3s Electron subshell recap 1s2 2 • Question: Write out the full electronic2p6 2s 2 for the element: Si 3p2 3ssubshell • 1s2 2s2 2p6 3s2 3p2 Si 1s2 2s2 2p6 3s2 3p2 Electron subshell recap 1s2 2 • Question: Write out the full electronic2p6 2s subshell for the element: Si • 1s2 2s2 2p6 3s2 3p2 Si 1s2 2s2 2p6 Electron subshell recap 1s2 2 • Question: Write out the full electronic2p6 2s subshell for the element: Si • 1s2 2s2 2p6 3s2 3p2 Si 1s2 2s2 2p6 Electron subshell recap 1s2 2 • Question: Write out the full electronic2p6 2s subshell for the element: Si • 1s2 2s2 2p6 3s2 3p2 Si [Ne] 3s2 3p2 What about Cl- Electron subshell recap 1s2 2 • Question: Write out the full electronic2p6 2s for the element: Si 2 3ssubshell 3p5 • 1s2 2s2 2p6 3s2 3p2 Cl 1s2 2s2 2p63s23p5 Electron subshell recap 1s2 2 • Question: Write out the full electronic2p6 2s for the element: Si 2 3ssubshell • 1s2 2s2 2p6 3s2 3p2 Cl 1s2 2s2 2p63s23p5 • Its Cl-1 • Which means it has an extra electron! Electron subshell recap 1s2 2 • Question: Write out the full electronic2p6 2s for the element: Si 2 3ssubshell • 1s2 2s2 2p6 3s2 3p2 Cl 1s2 2s2 2p63s23p5+1=6 Electron subshell recap 1s2 2 • Question: Write out the full electronic2p6 2s for the element: Si 2 3ssubshell • 1s2 2s2 2p6 3s2 3p2 Cl 1s2 2s2 2p63s23p6 • Have a go at potassium • And then K+1 (hint it has lost 1 electron). Now attempt the worksheet... Ions and metallic bonding To be able to work out the charge on different ions To know the properties of metallic bonding Ions • Ions are atoms or molecules that have lost or gained electron(s). • Positively charged ions are known as cations – Just think, cats are great, really positive • Negatively charged ions are called anions – No one likes onions... • Metals lose electrons to become positive (cations) • Non-metals gain electrons to become negative (anions). • If you see a Roman numeral after an element it usually means how many electrons it has lost. • Transition metals can promote electrons within sub shells to lose varying numbers of electrons. What charge will form? • The easiest way to predict how many electrons will be lost or gained is to use the periodic table. • Group 1 lose 1 electron • Group 2 lose 2 electrons • Group 6 gain 2 electrons • Group 7 gain 1 electron • This is because atoms are looking to fill/empty outer shells. Quick task • Can you predict how many electrons will be lost or gained by the following elements? –O – Cl – Al – Br – Na – Ca –H – Fe? • Have a go at the worksheet... Electron subshell recap • Question: Write out the full electronic subshell for the element: Si • 1s2 2s2 2p6 3s2 3p2 Metallic bonding • Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Even a metal like sodium (melting point 97.8°C) melts at a considerably higher temperature than the element (neon) which precedes it in the Periodic Table. • The simplest description of the bonding in metals consists of a lattice of metal cations in a sea of delocalised electrons. • A metal atom releases its outer electrons into the sea, but its core electrons remain localised around its nucleus. This extensive delocalisation of valence electrons is responsible for most of the properties of metals. • There is an attraction between the positive cations and the negative electrons. • The more negative electrons in the outer shell available to become delocalised, the stronger the bonding. • There the higher boiling point. • Which metal has a higher boiling point, sodium or magnesium? • What happens as you go down the group? • More shielding from inner shells, therefore less of a positive charge to attract to the delocalised electrons. • Therefore weaker intermolecular bonding • Therefore lower melting points. Diagram... Key definitions • Metallic bonding: The electrostatic attraction between positive metal ions and delocalised electrons.