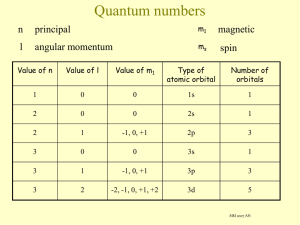
element
advertisement
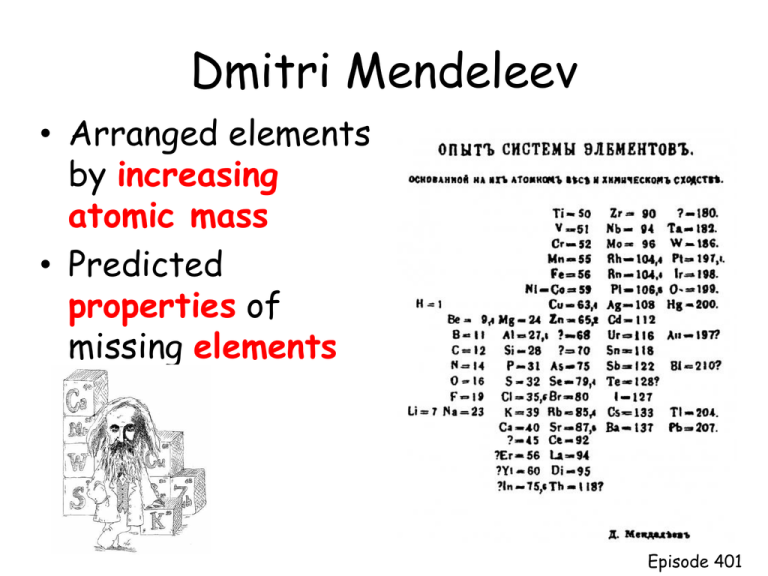
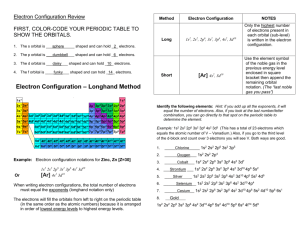
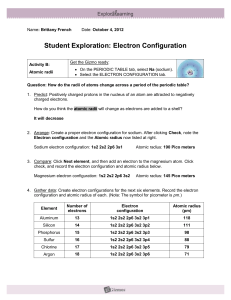
Dmitri Mendeleev • Arranged elements by increasing atomic mass • Predicted properties of missing elements Episode 401 Henry Moseley • Discovered that each element has a unique atomic number • Arranged elements by increasing atomic number • Now all the elements fit into place based on properties Episode 401 Modern Periodic Law • Properties of elements repeat when elements are arranged by increasing atomic number • Periodic function or repeats Episode 401 Questions about the extended periodic table: • Why do you think these elements get pulled out? – It makes the periodic table more compact. • Which element is in a different column than we are used to? – He • What happens to the number of energy levels as you begin each new row? – Increases by 1 • What is the similar characteristic in each column? – Electron distribution Episode 401 Elements in the same column: • Have similar electron distributions • Have same number of valence electrons – Electrons lost, gained, or shared – Found in the s and p orbital of highest energy level • Have similar chemical properties – Ne: • 1s2 2s2 2p6 – Kr: • 1s2 2s2 2p6 3s2 4s2 3d10 4p6 Episode 401 Noble Gases • Have full outer energy level • Have 8 valence electrons • Are stable – (inert or unreactive) Episode 401 Using the periodic table to find electron distributions: Episode 401 Noble Gas Configuration • Shorter way to do electron configurations using noble gases • Find noble gas on the end of row before the element and put its symbol in brackets. • Use diagonal rule to write the electron distribution for Sn (atomic # 50) – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2 • Use the noble gas distribution to write electron distribution for Sn – [Kr] 5s2 4d10 5p2 Episode 401 Noble Gas Configuration • Find noble gas on the end of row before the element and put its symbol in brackets. • Use diagonal rule to write the electron distribution for Fe (atomic # 26) – 1s2 2s2 2p6 3s2 3p6 4s2 3d6 • Use the noble gas distribution to write electron distribution for Fe – [Ar] 4s2 3d6 Episode 401