Document
advertisement

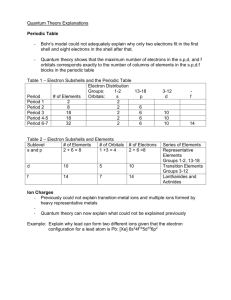
#1. Name 2 elements in which the last electron(s) to be added are placed into s subshells. You could have named any elements in group 1A or group 2A #2. How many electrons can be placed into the 2s subshell? 2 #3. Name 2 elements for which the last electron(s) to be added are placed into p subshells. You could have named any elements in group 3A, 4A, 5A, 6A, 7A or 8A #4. List all the elements with 6 electrons in the outermost p subshell. 8A: Neon, Argon, Krypton, Xenon, Radon #5. Name 2 elements for which the last electron(s) to be added are placed into the 3d subshell. Period 4, Groups 3-12 #6. How many electrons can be placed into the 3d subshell? 10 #7. Shade in the s-block, p-block, d-block and f-block with different colors of pen or pencil on the periodic table on the previous page. #8. A total of eighteen electrons can be placed in the 3rd shell. Explain why. 3s 2 3p 6 e 3d 10 e e ________ 18 e #9. How many subshells are in the 4th shell? 4: 4s, 4p, 4d, 4f #10. An electron configuration is a list of all the subshells that have electrons for a given element. Determine which element is associated with each electron configuration in the following table. Electron Configuration Element 1s22s1 Lithium, Li 1s22s22p3 •Nitrogen, N 1s22s22p63s23p5 •Chlorine, Cl 1s22s22p63s23p64s23d6 •Iron, Fe 1s22s22p63s23p64s23d104p2 •Germanium, Ge 1s22s22p63s23p64s23d104p65s24d105p4 •Tellurium, Te #11. Refer to the following electron configuration and answer the questions below: 1s22s22p63s23p64s23d5 a) How many total electrons does this element have? 25 b) What element is this? Manganese (Mn) c) How many shells are represented? 4 d) How many subshells are represented? 7 #13. Consider the noble gas shorthand notation in the table below: Element Valence electrons Lithium, Li Electron Configuration [He] 2s1 Potassium, K [Ar] 4s1 4s1 Scandium, Sc [Ar] 4s23d1 4s2 Zinc, Zn [Ar] 4s23d10 4s2 2s1 a) What do you think [He] and [Ar] stand for? The electron configuration up to that point. b) Use this shorthand notation to write the electron configurations for the elements in Group IIA, the alkaline earth metals). •Beryllium: [He] 2 •Magnesium: [Ne] 3s 2 •Calcium: [Ar] 4s 2 •Strontium: [Kr] 5s 2 •Barium: [Xe] 6s 2 2s c) Explain why the elements in Group IIA have similar properties. They all have 2 electrons in their outer shell. d) Use this shorthand notation to write electron configurations on your handout for the elements in the third row from sodium to silicon. 1 3s Na: [Ne] 2 Mg: [Ne] 3s 2 1 Al: [Ne] 3s 3p 2 2 Si: [Ne] 3s 3p e) Explain why the properties of the elements change as you move across the row. They have different #s of valence electron.