Topic 3 * The Periodic Table
advertisement
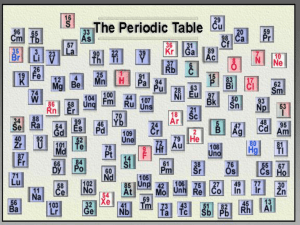
Topic 3 – The Periodic Table Syllabus Statements • In this topic you will study: • The Periodic Table • Physical Properties • The chemical properties of some elements and their oxides. 3.1 The Periodic Table • 3.1.1 Describe the arrangement of elements in the periodic table in order of increasing atomic number. • 3.1.2 Distinguish between the terms group and period. • 3.1.3 Apply the relationship between the electron arrangement of elements and their position in the table up to Z=20. • 3.1.4 Apply the relationship between the number of electrons in the highest occupied energy level for an element and its position in the periodic table. Because I know someone will ask, lets get this over with straight away • The trite phrase "the 92 naturally-occurring chemical elements" is often seen, but is incorrect. There are only 88 naturallyoccurring chemical elements. The elements 43, 61, 85 and 87 have no stable isotopes, and none of long half-life, so they are not naturally present. Small amounts are made in nuclear reactions induced by cosmic rays and nuclear tests, but these soon disappear. If you protest that these should be included, then so should Np and Pu, which are produced by the absorption of neutrons arising from spontaneous fission of uranium and thorium, and then there would be 94 naturally-occurring elements. If you wait long enough, there will only be 81 naturally-occurring elements, since everything beyond lead has only unstable isotopes, though some are of very long half-life, and have survived since the beginning, fathering their radioactive series. Any way you look at it, there are not just 92 naturally-occurring chemical elements. • Scientists like to make lists! • When scientists arranged all the known elements into order of increasing mass number, they found patterns in the reactions of the elements. • Certain properties seemed to repeat every 8 elements Atomic number and patterns reactive gases H He Li Be B C N O unreactive gases F Ne Na Mg Al Si P S Cl reactive metals Ar K • Further study and refinement led to the Periodic Table, which is one of the most useful tools we have in chemistry. • The modern periodic table arranges elements in order of increasing atomic number. • Note that we now use the number of protons NOT the mass number. • Why? • The proton number of an element always stays the same. (Remember isotopes!) • This order better reflects electronic structure (why?), which determines the reactions of an element. • The periodic table is organised into groups – a column of the periodic table • And periods – a row of the periodic table • Groups and periods can both be numbered • So we can talk about “the group 7 elements” • Or “the elements of period 2” • Note that hydrogen doesn’t really fit into a group. Sometimes it is placed on its own, and sometimes it is placed at the start of group 1 Columns of elements 1 2 3 Groups 4 5 6 7 H Li He Be Ca Sc Rb Sr B C N O F Ne Al Si P S Cl Ar Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr transition elements Na Mg K 0 Y Ti V Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Cs Ba La Hf Ta W Re Os Ir Fr Ra Ac Rf Db Sg Bh Hs Mt I Xe Pt Au Hg Tl Pb Bi Po At Rn ? ? ? Rows of elements Periods 1 H 2 Li Be B C N O F Ne 3 Na Mg Al Si P S Cl Ar Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 4 K He Ca Sc Ti V 5 Rb Sr 6 Cs Ba La Hf Ta W Re Os 7 Fr Ra Ac Rf Db Sg Bh Hs Mt Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Ir I Xe Pt Au Hg Tl Pb Bi Po At Rn ? ? ? • A few more things to note at IB level: • Some textbooks (and old doddery teachers like me) sometimes say group 8 instead of group 0. They are the same group. Correct me if I get it wrong! • Sometimes the transition elements are given group numbers from 3 to 12. • Then the old group 3 becomes group 13; the old group 4 becomes group 14 etc. • The avoid any confusion the IB textbook refers to group 3/13; group 4/14 and so on. • Its easy to understand – it’s just a bit annoying. Group or Family Period or Series The Periodic Table Group or family Period • Notice that chemists have given some groups names. • Group 1 are the alkali metals (why?) • Group 2 are the alkali earth metals (but these aren’t studied at IB) • Group 7 are the halogens • Group 0 (or group 8) are the noble gases. • Thinking back to the Atomic Structure topic, what is the relationship between proton number and number of electrons? • They are the same. • So the periodic table can be used to deduce the electron structures of all the elements. • You should already know the first 20. Element Lithium Carbon Argon Calcium Boron madeupium doesntexistium Number of electrons in valence shell Group number 4 3 Element Lithium Carbon Argon Calcium Boron madeupium doesntexistium Number of electrons in valence shell Group number 1 4 8 2 3 4 3 1 4 8 2 3 4 3 • It should be really really obvious that the number of electrons an element has in its outer shell tells us what group the element is in. • This applies even to elements you haven’t heard of (not just the 20 you are supposed to have learned!) • How many electrons are in the outer shell of Gallium, Ga • It’s in group 3, so it has 3 electrons in its outer shell • Less obvious, but still important is the relationship between the period an element is in and the number of electron shells it has. • • • • Lithium is in period 2 Its electron configuration is 2,1 So it has 2 occupied shells. 2 electrons in the first shell and one electron in the second shell • Its highest occupied shell is shell number 2 • Potassium has the electron configuration 2,8,8,1 • It has 4 occupied shells, so it must be in period 4 • Its highest occupied shell is shell 4. • Note that sometimes we refer to an electron shell as an “energy level” Review of syllabus statements • 3.1.1 Describe the arrangement of elements in the periodic table in order of increasing atomic number. • 3.1.2 Distinguish between the terms group and period. • 3.1.3 Apply the relationship between the electron arrangement of elements and their position in the table up to Z=20. • 3.1.4 Apply the relationship between the number of electrons in the highest occupied energy level for an element and its position in the periodic table.