Electrochemistry - Winston Knoll Collegiate
advertisement

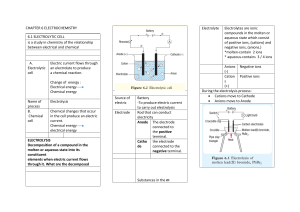
Electrochemistry Electrochemistry deals with the relation of the flow of electric current to chemical change, and the conversion of electrical and chemical energy. There are two main branches: electrolysis electrochemical cells Electrolysis is a non-spontaneous reaction where electrical energy is converted to chemical energy. Common examples: metal plating (silver, gold, rhodium) decomposition of water refining of pure metals and chemicals charging a battery In an electrochemical cell, a spontaneous reaction occurs where chemical energy is converted to electrical energy. Common examples Component Electrodes car battery dry cell (acidic or alkaline) rechargeable batteries galvanic corrosion Electrolytic Device Electrochemical Device usually a metal, but may be another conductor such as graphite Anode the electrode at which oxidation takes place has positive charge outside current forces Cathode the electrode at which reduction takes place has negative charge Electrolyte Curcuit has positive charge ions dissolved in a water solution ions in melted ionic solid External has any negative charge includes device that moist paste electrons flow anode to cathode not spontaneous Rx’n spontaneous Rx’n Electrolysis - is a process by which an external electric current causes a redox reaction to occur in a water solution of an electrolyte or in a pure electrolyte in the liquid phase. The reaction will not occur without the external circuit. There are 3 essential parts to an electrolytic device: - a source of direct current - 2 electrodes, the cathode (negative) and anode (positive) - an electrolyte The negative anions in the electrolyte are attracted to the anode. And the positive cations are attracted to the cathode. Electrons are lost at the anode (LEO) and gained at the cathode (GER). Electroplating In the process of electroplating (silver), the object to be plated (a spoon) is set up as the cathode. This is so the negative charge of the electrode will attract the positive metal (silver) ions in solution. The electrolyte must be a soluble salt of the metal (silver nitrate), and the anode must be a solid strip of the metal being plated. The strip’s function is to replace the positive ions in solution that have “plated out”. - + Rx’n at the anode (strip) : Ag(s) ➩ Ag+ + e- Ag+ NO3- Power Source + + + - Rx’n at the cathode (spoon): Ag+ + e- ➩ Ag(s) The silver ions formed at the anode replace those from the electrolyte that are plated on the spoon. Thus the anode loses mass and the cathode gains in mass. Decomposition of Water An electric current is applied to water which decomposes it into the elements hydrogen and oxygen. Rx’n at the anode (LEO) Rx’n at the cathode (GER) 2H2O ➩ O2 + 4H+ + 4e- (acidic) 4H2O + 4e- ➩ 2H2 + 4OH- (basic) 6 H2O ➩ 2H2 + O2 + (4H+ + 4OH-) 2H2O ➩ 2H2 + O2 Electrolysis of Molten Sodium Chloride An electric current is passed through molten (liquid) sodium chloride. The result is pure sodium metal and pure chlorine gas. Rx’n at the anode (LEO) Rx’n at the cathode (GER) 1(2Cl- ➩ Cl2(g) + 2e-) 2(Na+ + e- ➩ Na(s)) 2NaCl ➩ 2Na(s) + Cl2(g) Any pure metal can be obtained from its soluble salt using a similar process. Na+ + e- ➩ Na(s) Ni2+ + 2e- ➩ Ni(s) Al3+ + 3e- ➩ Al(s) Notice that each metal must gain a different number of electrons. The correct amount of electricity must be calculated and carefully supplied to have maximum effectiveness.