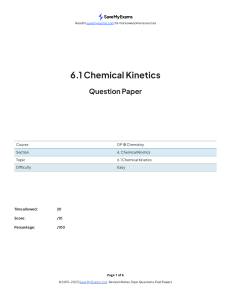
KINETICS 5 Factors 1. Temperature 2. Concentration of Solution which influence the rate Remember if Generally Emperature by 3. Pressure of Gas 4. Surface 5. Catalyst area the As temperature therefore D. As or is himhis an energy in increase increases, concentration than double increases, rate more there more will of solid have increase 1002, this we * particles of reachin reaction of they so increases as faster and more frequent successful number activation energy to rate of reaction inverses particles of per unit thereforethere ismore fregret success collisions C collisions equal to of volume. in the is rig 3.Aspressure increases there aremoreparties acceptin the in right D. As Surface collide surface the 5. orientation Area with right so increases, more other rectant as there are orientation. more particles more will be available to particles exposed frequent successful at the collisions in will speed up the reaction without being chemically catalyst used up. and it does They lower the required Activation Energy love reaction that has alternate pathway for thisbyproviding A an a a one gnere Uncatalysed reaction ↳ - caralysedthe ↳ime of Reaction Measuring Rate Rate Units · = unit / Products) m0ldm 5- The Rate of in [Reactants Time Change = of Reaction the rectants of or is the time, the measure change in of the concentration change of the in concentration products per Collision Theory - Chemical reaction reactions to greater and the occur than occur when successfully, or particle equal to orientation particles of these substances collide. For collisions must have activation energy must be correct. the of a energy reactio the Asrate of reaction increases, the number of particle with ears