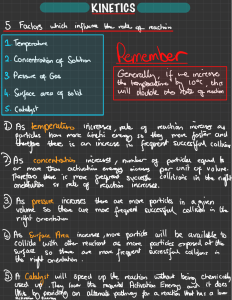
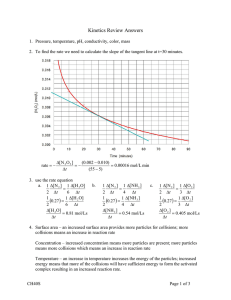
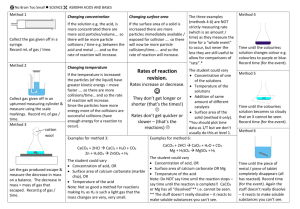
Rates of Reaction The rate of a reaction is determined by how many successful collisions occur each second. The more collisions with enough energy, the more reactions will be occurring. 4 Things influence the Rate of Reaction Temperature Concentration Surface Area Catalyst We observed the particles in the hot water….. In comparison the cold water….. Having more acid particles in the same volume results in more collisions between acid and potassium permanganate particles each second. This is why …..