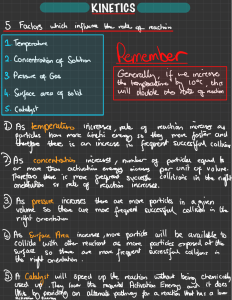
RATES OF REACTION Reaction rate is a measure of how fast a reaction takes place. Several factors can affect the rate of a reaction. They include: Concentration of the reactant solutions. Pressure of the reactant gases. Temperature at which the reaction is carried out. Surface area of solid reactants. Use of a catalyst. Pressure of the reactant gases Increasing pressure pushes the gas particles close together so that they collide and react more readily, increasing the reaction rate. An example of a reaction that shows effect of increasing pressure is the combustion of fuel in a car engine as the movement of piston compresses them. The curve of the reaction done at a high pressure has a steeper gradient and becomes horizontal sooner. Surface area of solid reactants In reactions involving solid reactants, the more finely divided a solid reactant is, the greater the rate of reaction. Reactions involving solids take place on the surface of the solid. Dividing/powdering a solid increases its surface area. Experiment. Reaction between calcium carbonate and hydrochloric acid. CaCO3 + HCI CaCI2 + CO2 + H2O Production of CO2 results in a loss in mass. When finely divided calcium carbonate is used, carbon (IV) oxide is produced at a faster rate than when larger particles of calcium carbonate are used. Collision theory of the reaction rates Collision theory states that for a chemical reaction to take place, particles of the reactants must collide with sufficient energy to initiate a reaction i.e. enough energy to break bonds. Activation energy (Ea) The minimum amount of energy that colliding particles must have to react is known as the activation energy. Collisions that result in a reaction are known as successful collisions. Such collisions have sufficient energy (i.e. energy greater than the activation energy). The number of successful collisions increase with increasing temperature. Not all collisions result in a chemical reaction. Most collisions just result in the colliding particles bouncing off each other. Collisions that do not result in a reaction are known as unsuccessful collisions. Unsuccessful collisions happen when the colliding species do not have enough energy to break the necessary bonds (i.e. they collide with energy less than the activation energy) Increasing the number of successful collisions means that a greater proportion of reactant particles collide to form products. Factors affecting the number of successful collisions The number of particles per unit volume - more particles in a given volume will produce more frequent successful collisions. A more concentrated solution has more reactant particles per unit volume. This means that increasing the concentration increases the rate of a chemical reaction. The frequency of collisions - a greater number of collisions per second will give a greater number of successful collisions per second. The kinetic energy of the particles - greater kinetic energy means a greater proportion of collisions will have an energy that exceeds the activation energy and the more frequent the collisions will be as the particles are moving quicker; therefore, more collisions will be successful. The activation energy – only collisions that will have an energy that exceeds higher activation energy will be successful. These all have an impact on the rate of reaction that is dependent on the number of successful collisions per unit of time. Explaining rates of reaction using collision theory Temperature Increasing the temperature will increase the rate of reaction. This is because the particles will have more kinetic energy than the required activation energy. Therefore, there will be more frequent collisions and a higher proportion of particles will have energy greater than the activation energy. This causes more successful collisions per second, increasing the rate of reaction A small increase in temperature causes a large increase in the rate of a reaction. Concentration Increasing the concentration of a solution will increase the rate of reaction. This is because there will be more reactant particles in a given volume, allowing more frequent and successful collisions per second, increasing the rate of reaction. For example, concentrated HCI has a higher rate of reaction with Magnesium than dilute HCI. Pressure For a gaseous reaction, increasing the pressure brings the particles much closer increasing their chances of collision, creating a greater chance for a reaction to occur. Surface area Increasing the surface area of a solid will increase the rate of reaction. This is because more surface area of the particles will be exposed to the other reactant, producing a higher number of collisions per second increasing the chance of a reaction taking place. Catalysts Catalysts are substances that speed up the rate of a reaction without themselves being altered or consumed in the reaction. The mass of a catalyst at the beginning and end of a reaction is the same and they do not form part of the equation. Catalysts increase the rate of reaction by reducing the amount of energy that is needed to break the bonds (activation energy) This means a higher proportion of the reactant particles will have energy greater than the activation energy and will result in more successful collisions per second An important industrial example is when iron is used to catalyze the Haber process for the production of ammonia. Iron beads are used to increase the surface area available for catalysis. Enzymes are biological catalysts, they work best at specific temperature and pH ranges. Normally only small amounts of catalysts are needed to have an effect on a reaction.