Electron Configuration Worksheet
advertisement
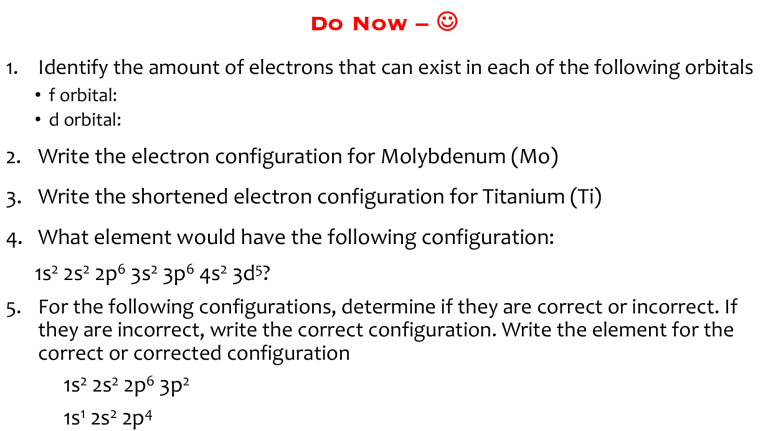
Do Now – J 1. Identify the amount of electrons that can exist in each of the following orbitals • f orbital: • d orbital: 2. Write the electron configuration for Molybdenum (Mo) 3. Write the shortened electron configuration for Titanium (Ti) 4. What element would have the following configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d5? 5. For the following configurations, determine if they are correct or incorrect. If they are incorrect, write the correct configuration. Write the element for the correct or corrected configuration 1s2 2s2 2p6 3p2 1s1 2s2 2p4 6. Give an example of an excited state electron configuration for: a. Mg b. He c. P 7. Is the electron configuration an excited or ground state? a. 2-8-4 b. 2-3-1 c. 2-8-7-1 8. Consider the diagram below, which shows bright-line spectra of selected elements. a. Identify the two elements in the unknown spectrum. b. Explain how a bright-line spectrum is produced, in terms of electrons and energy states.