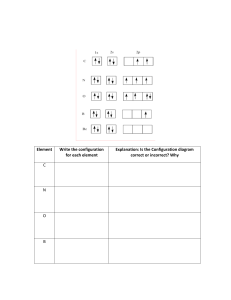
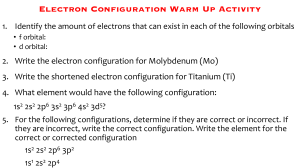
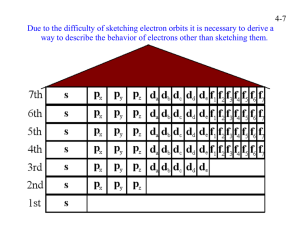
PRE-TERTIARY ELECTRONIC CONFIGURATION DIAGONOSTIC TEST (PRECDT) PRE-TEST Purpose:This pretest seeks to find out your conceptual understanding of electronic configuration before flipped classroom approach (FCA) intervention activities. This exercise is being conducted for the research purpose only and because of this marks obtained on the test will be treated confidentially. Thank you for co-operation. BIO-DATA OF THE RESPONDENT GENDER: Male Female Age Boarder Day Instruction This test consists of two sections; the section A & B. For section A, circle the correct option to each item, for B provide your candid answers in the spaces provided. Answer all the questions on this paper for marks. Duration minutes. SECTION A OBJECTIVE TEST 1. The total number of electrons that can occupy the principal energy level is A. 2 B. 8 C. n D. 2n2 2. For an electron with angular quantum number l = 2, the magnetic quantum number can have A. An infinite number of values B. Only one value C. One of two possible values D. One of five possible values 3. An electron cannot have the quantum numbers n = ,l= ml = A. 2, 0, 0 B. 2, 1, 1 C. 3, 1, - 1 D. 1, 1, 1 4. Which one of the following is an impossible subshell notation A. 4f B. 2d C. 3s D. 2p 5. A 3p can have possible magnetic quantum number of values of A. 3 and 6 B. -2, -1, 0 and 1 C. 3, 2 and 1 D. -1, 0 and 1 6. Which electronic configuration represents a violation of the Pauli’s exclusion principle A. 1s 2s 2p B. 1s 2s 2p C. 1s 2s 2p D. 1s 2s 2p 7. What is the correct electronic configuration for Mg2+ A. 1s2 2s2 2p6 B. 1s2 2s2 2p6 3s2 C. 1s2 2s2 2p5 3s1 D. 1s2 2s2 2p4 3s2 8. A certain transitional metal has an electron configuration such that its 4s orbital only has one electron. What would be a valid conclusion about this element A. This is an invalid electronic configuration because 4s orbital always contain two electrons in transitional metals B. The identity of the element is Manganese and it contain an empty 3d orbital C. The identity of the element is Chromium and it contain one electron in each 3d orbital D. The identity of the element is Manganese and it contains one electron in each 3d orbital 9. An atom with electronic configuration 1s2 2s2 2p6 could be any of the following except A. Ne B. Mg2+ C. FD. Na 10. Pauli’s exclusion principle is related to A. Quantum numbers of electrons B. Filling degenerate orbitals C. Filling the orbitals with lower energy first D. Quantity of electrons in the valence shell 11. The Bohr’s model of the atom proposed the existence of A. The nucleus B. Nucleons C. Electron shells D. Neutrons 12. Which of the following species is represented by the electronic configuration 1s2 2s2 2p6 3s2 3p6 3d5 4s1 A. Fe B. Cr C. Mn D. Mo 13. How many orbitals are contained in an atom with atomic number 13 A. 7 B. 6 C. 5 D. 13 14. The electron configuration of Nitrogen is represented as 1s 2s 2p 1s and not 2s 2p This is in accordance with A. Quantum theory B. Pauli’s Exclusion principle C. Hund’s Rule of Maximum multiplicity D. Aufbau principle 15. Which of the following statements is true A. An electron in an n = 1 shell has lower energy than an electron in an n =2 shell B. All subshells contain same number of electrons C. The number of subshells within a shell is equal to the orbital number D. All subshells have an identical shape and size SECTION B 16. Indicate if anything is wrong for each of the following ground state electronic configuration 3d 4s 4p a. 3d 4s 4p 3d 4s 4p 3d 4s 4p b. c. d. 3d 4s 4p e. 17. How many electrons can be accommodated in the following a. b. c. d. e. A d subshell A set of f orbitals The n = 4 shell The 7s orbital A p orbital 18. The electronic configuration of oxygen is as follows 1s 2s 2px 2py 2pz a. Explain briefly the significance of the i. Arrows j. letters x, y and z b. Why is the electron in the 2pz orbital not located in the 2py orbital 19. How many sublevels does Neon atom have 20. Write a. The detail electronic configuration of Cr3+ b. State the number of unpaired electrons