Chm 123 Name: Evaluation Quiz
advertisement

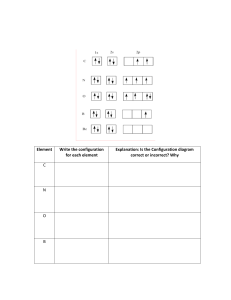
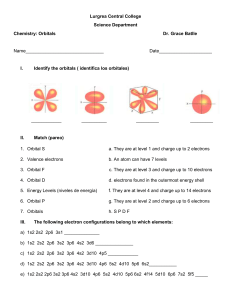
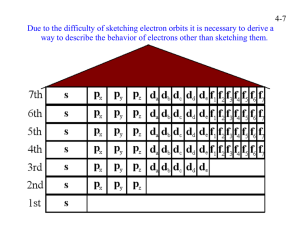
Chm 123 Evaluation Quiz 00 Points Name: 1. Write the electron configuration of nitrogen, and copper. N 1s2 2s2 2p3 Cu 1s2 2s2 2p6 3s2 3p6 3d9 4s2 (or 3d10 4s1) 2. What is the approximate H-C-H bond angle in CH4? What is the molecular shape of CH4? bond angle approx. 109.5 degrees, tetrahedral 3. For the Lewis structure below draw a resonance structure and indicate both the formal charge on any charged atom and the overall molecular charge. Circle the resonance structure you drew if it is more stable than the structure shown. 4. Write the product of each chemical reaction. Identify each component as either an acid or a base. 5. Provide a name (either IUPAC or common) for the following compounds. Circle any compounds that contain ONLY covalent bonds. Sodium bromide Potassium hydroxide Acetic acid Ethanol Ammonium carbonate