Chemistry Ionic Compounds Assignment: Module 1, Lesson 3
advertisement

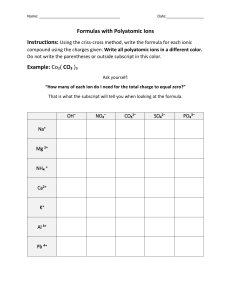
Chemistry 20: Module 1: Lesson 3 1 Assignment MODULE 1: LESSON 3 ASSIGNMENT This Module 1: Lesson 3 Assignment is worth 50 marks. The value of each assignment and each question is stated in the left margin. (29+9=38/50 Marks) Lesson 3 Assignment: Ionic Compounds (16+8=24/30 marks) 1. Fill in the table with the required information. The first one is done for you. Atoms Combined Formula of Ions Involved Chemical Formula Name of Compound Na and Cl Na+ and Cl– NaCl sodium chloride Ca and Cl K and Br CaCl2 You need the Ca2+ AND Clcorrect charges first What is the before you can common ion charge criss cross them of Ca? Look on the and write the periodic table formula calcium chloride K+ and Br- KBr KBr potassium bromide Fe3+ and (OH)– Fe2(OH)3 You need to keep the polyatomic ion in the brackets and then criss cross the charges iron (III) hydroxide Al and S Al3+ and S2Use the periodic table to check the charges Al2S3 You need the correct charges first before you can criss cross them and write the formula aluminium sulfide Ni and CN Ni3+and (CN)What is the charge of Ni? And the charge of CN? It is in the polyatomic ion chart Ni(CN)3 nickel(II) cyanide Ga2(SO4)3 • 18H2O GALLIUM(III) SULFATE OCTADECAHYDRATE FE AND OH- Ga3+(SO4)2- and H2O GA(SO) and H2O What is the charge of Ga? And the charge of SO4? It is in the polyatomic ion chart Chemistry 20: Module 1: Lesson 3 2 Assignment Cu2+AND N3Use the periodic table to check the charges Cu3N2 copper (II) nitride Mg2+ and O2– MgO Yes but you can divide both charges by 2 (lowest common number) and then you get Mg1O1 or MgO magnesium oxide Al and O Al3+ AND O2Use the periodic table to check the charge of Al Al2O3 You need the correct charges first before you can criss cross them and write the formula aluminium (II) oxide Not 2 (II) Au+ AND BO Au2+ AND BO3 What is the charge of Au? And the charge of BO3? It is in the polyatomic ion chart Au3BO2 gold (II) borate Cu AND N MG+ AMD O- Fe3+ and OH1OH is a poly atomic ion, it needs brackets Fe3+ and (OH)1now criss cross the charges Fe1(OH)3 FeOH3 = 1 Fe, 1 O, 3 Hs Fe(OH)3 = 1Fe, 3Os, 3Hs Ionic hydrates are on page 3 of your lesson, pg 31 in the textbook (19+2/30=13+1/20 marks) 2. Complete the following table. Element Electronegativity Group Number Number of Valence Electrons Number of Bonding Electrons Number of Lone Pairs of Electrons Mg 1.3 2 2 2 0 Cl 3.2 17 7 1 6 Chemistry 20: Module 1: Lesson 3 3 Assignment Al 1.6 13 3 3 0 Na 0.9 1 1 1 10 Si 1.9 14 4 4 4 H 2.2 1 1 1 0 P 2.2 15 5 3 1 Bonding electrons and Lone pairs - see page 91 Figure 2 Let’s do the first one together Given 2 valence electrons, so that means it’s in group #2 (alkaline earth metals), with 2 bonding electrons – no lone pairs, look up 1.3 on the periodic table of group 2 and you will find Mg has 1.3 Once you have completed all of the questions, submit your work to your teacher.