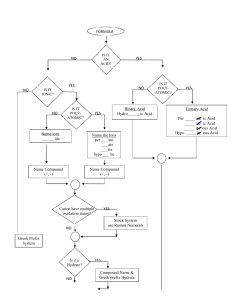
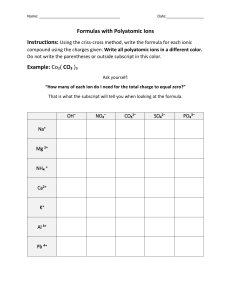
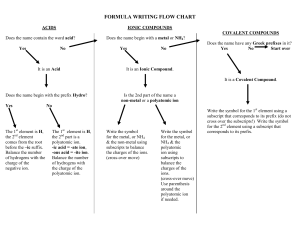
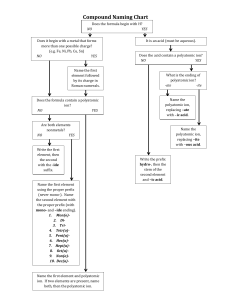
Notes on Polyatomic Ions _______________and have an overall ________________.
advertisement

Notes on Polyatomic Ions Polyatomic Ion – _________________ atoms bonded together that act as an _______________and have an overall ________________. Can act as a ___________or a ________________ ion. Rules: 1. If more than one group is needed to balance a formula, the group must be enclosed in parenthesis before adding the subscript. Example: 2. Patterns: ate the standard version of the ion Example: ite 1 less oxygen that ate. The charge does not change depending on how many oxygen atoms are involved Example: per 1 more oxygen than ate Example: hypo 1 less oxygen that ite. Hypo means below. Example: 3. Patterns: ates with charges less than -1 can have hydrogen added to them. For each hydrogen added the charge is increased by +1 Example: