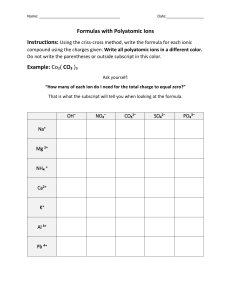
SCH3U Grade 10 Chemistry Review This package should serve to help jog your memory of grade 10 science. Parts of this package will be explanation; other parts will be engaging you. Your teacher will work through this package with you. Section A (recall) ✓ Matter – Substances vs. Mixtures ✓ Physical vs. Chemical Properties ✓ Atomic Structure ✓ Evidence of Chemical Reactions o There is an unexpected change in colour o A gas is produced o Energy is released or absorbed o A precipitate forms Section B ✓ Counting Atoms ✓ Law of Conservation of Mass Section C ✓ Naming Ionic & Molecular Compounds and Writing Chemical Formulas Section D ✓ Balancing chemical reactions ✓ Identifying Reactions Section E: Writing Balanced Chemical Reactions ------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 1 SCH3U Section B Counting Atoms The subscripts will tell you how many atoms of each element there are. If there are brackets – multiply the subscript outside of the brackets with the subscript inside If there is a coefficient – multiply the coefficient with the subscript of the element (and the subscript outside the brackets if brackets exist) Example: Type of Atom 2Mg(OH)2 # of Atoms Total Now practice on the Counting Atoms Worksheet Law of Conservation of Mass: The law of conservation of mass states that during a chemical reaction, the total mass of reactants equals the total mass of products. LS = RS # of elements = # of elements PRACTICE ON HANDOUT P 27 Section C: Naming Ionic & Molecular Compounds and Writing Chemical Formulas IONIC COMPOUNDS: Given Formula, Write the Name Points to remember about naming ionic compounds 1. Write the cation (positively charged atom, usually a metal) first, then the anion (negatively charged atom, usually nonmetal) 2. Use the name of cation (metal) with a fixed oxidation state (common charge) directly from the periodic table. 3. The name of the anion will be made from the root of the element's name plus the suffix "-ide." Write the correct name for: 1. MgS 2. MgF2 3. Sr3P2 4. K2S 2 SCH3U PRACTICE ON HANDOUT P 45 Let’s go backwards: Given name, write chemical formula 1. 2. 3. 4. The metal comes first, then the nonmetal. Use your periodic table to find out the charges of each metal and nonmetal. The sum of the positive charge and the sum of the negative charges MUST add up to zero. Use the “criss-cross” method. a. write out both element’s symbols with their charges b. “criss-cross” the numbers ONLY c. reduce to lowest ratio, if needed Example Write the formula for cesium oxide. (Here you will need to use the lowest ratio) 1. Write Cs+ and O2¯ right next to each other. 2. The least common multiple between the two charges is 2. 3. This means that +2 is the total positive charge and -2 is the total negative charge needed. 4. Two Cs and one O each provide the needed amount of charge, so the correct formula is Cs2O. (i.e. adjust the subscripts to get a total charge of zero) 1. aluminum nitride 2. beryllium bromide 3. barium iodide 4. boron phosphide Binary Compounds of Metals with Variable Charges: Given Formula, Write the Name The metals involved have AT LEAST TWO possible charges. The nonmetals involved have only one charge. In most cases, simply “uncrossing” the subscripts will work. But in the case of metals whose charges can be BOTH 2+ and 4+, a more rigorous method is needed. Write the name for: PbO2 Steps 1. The first part of the name is the unchanged name of the first element in the formula. 2a. Multiply the charge of the nonmetal by its subscript. Ignore the fact that it is negative. 2b. Divide this result by the subscript of the metal. This is the value of the Roman numeral to use 3. The value of the Roman number equals the positive charge on the metal in this formula. Write the full name using the Roman number. Solution O2Charge 2, subscript 2 subscript on Pb is 1 2×2=4 4÷1=4 Lead(IV)oxide 3 SCH3U Write the correct name for: 1. CuS: _____________ 2. SnO2 : _____________ 3. Hg2O : _____________ 4. Mn2O3: _____________ Given Name, Write the Formula 1. iron(II) chloride: _____________ 2. cobalt(III) phosphide: _____________ 3. chromium(II) nitride: _____________ 4. lead(IV) nitride: _____________ PRACTICE IONIC COMPOUNDS: NAMES & FORMULAS WORKSHEET USING TRANSITION METALS & HANDOUT P44 MOLECULAR COMPOUNDS: Given Formula, Write the Name Greek prefixes are used: one two three four five monoditritetrapenta- six seven eight nine ten Example #1 - write the name for N2O. Steps 1. Write the name of the first element. If subscript is 2 or more, add a prefix to the name. DO NOT use a prefix if the subscript is 1. 2. Write the second element in the same way as ionic compounds → “ide”. Also add a prefix. hexaheptaoctanonadeca- Solution dinitrogen monoxide Example #2 - write the name for NO2. ___________________ 1. sulfur tetrachloride: ______________ 3. chlorine monoxide: _____________ Given Name, Write the Formula 2. diphosphorus pentoxide __________________ 4. xenon hexafluoride: _____________ PRACTICE MOLECULAR COMPOUNDS: P46 Compounds Involving a Polyatomic Ion Given Formula, Write the Name These ARE NOT binary compounds. They contain three or more elements, as opposed to only two in a binary compound. If you see a formula like BaSO4, the name is not barium monosulfur tetraoxide. Learn to recognize the presence of a polyatomic ion in a formula and know its charge. The cations used will be a mix of fixed charges AND variable charges. You must know which are which. Use of Brackets When more than one polyatomic ion is required, put it in brackets and leave subscript outside. 4 SCH3U Example Fe(NO3)2 → two NO3¯ Without the parenthesis, the formula would be FeNO32 When you say a formula involving parenthesis out loud, you use the word "taken" as in the formula for ammonium sulfide, which is (NH4)2S. Out loud, you say "N H four taken twice S." OR with the formula for copper(II) chlorate, which is Cu(ClO3)2. You say " Cu Cl O three taken twice." Example 1 -Write the name for Fe(NO3)2 Steps 1a. Decide if the cation is one showing variable charge. If so, a Roman numeral will be needed. 1b. Compute the total charge contributed by the polyatomic ion Solution Fe shows variable charge NO3¯ has a minus one charge and there are two of them, making a total of minus 2. Fe must be a positive two, in order to keep the total charge of the formula at zero. nitrate iron(II) nitrate or ferrous nitrate 2. Determine name of polyatomic ion Write the name of the following polyatomic compounds: 1. AlPO4 : _____________ 2. Mg(OH)2 : _____________ 3. Cu(ClO3)2 : _____________ 4. manganese(II) sulfate: _____________ 1. lithium carbonate : _____________ Given Name, Write the Formula 2. tin(II) hydroxide: _____________ 3. silver phosphate: _____________ 4. cobalt(III) nitrate: _____________ Acids: Given Formula, Write Name Acid formulas can be recognized by the element hydrogen present at the beginning of the formula. i.e. HCl(aq), H2S(aq), HNO3(aq), H2SO4(aq) 1) If the acid has only ONE element besides hydrogen, it is called a binary acid and the name will follow the pattern: hydro(element root)ic acid e.g. HCl(aq) is hydrochloric acid 2) If the acid has a polyatomic ion in its formula, it is called an oxyacid and the name will follow the pattern: (element root)ic acid e.g. HNO3(aq) is nitric acid Write the name of the following acids: 1) HCl(aq) 1) hydrochloric acid 8) HNO3(aq) 8) nitric acid 2) HClO3(aq) 2) chloric acid 9) H2Se(aq) 9) hydroselenic acid 3) H2S(aq) 3) hydrosulfuric acid 10) HBrO3(aq) 10) bromic acid 5 SCH3U 4) HBr(aq) 4) hydrobromic acid 11) H3PO4(aq) 11) phosphoric acid 5) H2CO3(aq) 5) carbonic acid 12) HI(aq) 12) hydroiodic acid 6) HF(aq) 6) hydrofluoric acid 13) H2SO4(aq) 13) sulfuric acid 7) HIO3(aq) 7) iodic acid Given Name, Write the Formula 1) If the acid starts with the prefix hydro- then the acid formula can be obtained by combining H+ with the corresponding single-atom anion following the same method as ionic formulas. Acid formulas should always indicate that they are dissolved in water by using the state symbol (aq). e.g. hydrosulphuric acid H+ S2- H2S(aq) 2) If the acid does not contain the prefix hydro, the formula can be obtained by combining H+ with the corresponding polyatomic anion following the same method as ionic formulas. Acid formulas should always indicate that they are dissolved in water by using the state symbol (aq). e.g. phosphoric acid H+ PO43- Write the formulas for the following acids 1) hydrochloric acid 1) H3PO4 (aq) 8) sulphuric acid 8) 2) carbonic acid 2) 9) hydroiodic acid 9) 3) iodic acid 3) 10) phosphoric acid 10) 4) hydrobromic acid 4) 11) bromic acid 11) 5) chloric acid 5) 12) hydroselenic acid 12) 6) hydrofluoric acid 6) 13) nitric acid 13) 7) hydrosuphuric acid 7) Bases Bases are very easy to recognize - they usually contain the hydroxide anion. There are, of course, always exceptions to this rule. there are two bases that do not have hydroxide anion in their formula. They are NH3 (ammonia) and any metal carbonates (e.g. Na2CO3, K2CO3, MgCO3) Examples sodium hydroxide: NaOH sodium carbonate: Na2CO3 6 SCH3U Section D: Balancing chemical reactions Guidelines for Balancing Equations: 1. Write the skeleton equation, and make sure that you have written the formula for each molecule correctly. 2. Balance the atoms that occur in the largest number on either side of the equation first. Leave H, O and other elements until the end. 3. Balance polyatomic ions of both sides of the equation as a unit. 4. Balance other uncombined atoms and elements such as Na and Br2 5. Balance H and O atoms. 6. CHECK YOUR ANSWER. Remember that your coefficients should be in lowest terms. Balance the following equations: SF4 (g)+ H2O(l) → C3H8(g) + O2(g) SO2(g) + → CO2(g) + Al(s) + H2SO4(aq) → Al2(SO4)3(aq) + H2(g) HF (aq) H2O(g) PRACTICE ON HANDOUT P487 Section D: Identifying Reactions Type of Reaction Pattern synthesis decomposition single displacement double displacement complete hydrocarbon combustion combustion of an element neutralization A + B → AB AB → A + B A + BC → AC + B (A = metal) A + BC → BA + C (A = nonmetal) AB + CD → AD + CB CxHy + O2 → CO2 + H2O + energy A + O2 → AO + energy acid + base → salt + water (not all follow this → carbonates as bases) 7 SCH3U Example Al2(SO4)3 + 3Ca(OH)2 → 2Al(OH)3 + 3CaSO4 2H2O2 → 2H2O + O2 PRACTICE ON HANDOUT P488 8 SCH3U Section E: Writing Balanced Chemical Reactions Example sodium nitrate + barium sulphate → Steps Solution 1. Use the Type of Reactions chart Sodium nitrate + barium sulphate → sodium sulphate + barium above to predict the products nitrate 2. Write the elements with their Na+ NO3- + Ba2+ SO42- → Na+ SO42- + Ba2+ NO3charges for each compound 3. Use the rules above for writing Na NO3 + BaSO4 → Na2SO4 + Ba(NO3)2 formulas given names 4. Balance the equation 2Na NO3 + BaSO4 → Na2SO4 + Ba(NO3)2 Example barium + nitrogen → Example sodium hydroxide + hydrochloric acid → 9 SCH3U Practice: Identify the type of reaction, predict the product based on the reaction pattern, write the word equation and balanced chemical reaction. 1. ___Ag + ___Cl2 → 2. ___K + ___H2O → 3.___Fe +___H2SO4→ 4. ___CuSO4 + ___BaCl2 → 5. ___Zn + ___H3PO4 → __ 6. ___Al(NO3)3 + ___NaOH → 7. ___C25H52 +___O2 → 8. ___Cu + ___AgNO3 → 9. ___Cu +___H2O → 10. ___Cu +___Cl2→ 11. ___Mg +___N2→ 12. ___C +___O2 → 13.___NaOH + ___H2SO4 → 14. ___Al +___H2SO4→ 15. ___C2H6 +___O2→ 10