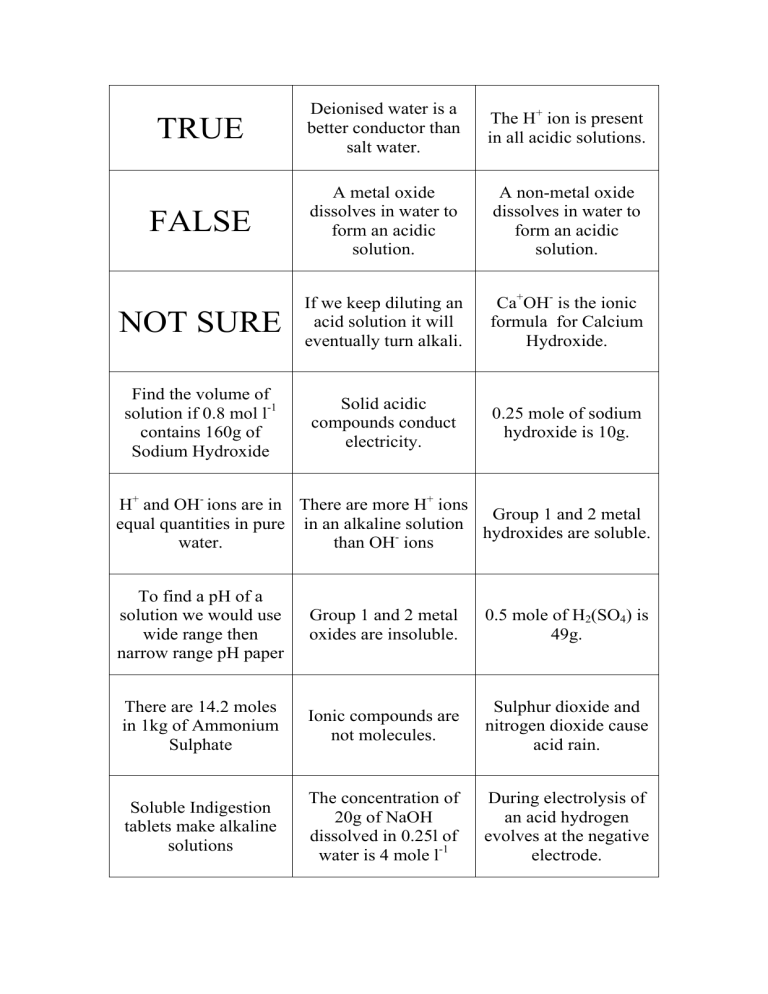
TRUE Deionised water is a better conductor than salt water. The H+ ion is present in all acidic solutions. FALSE A metal oxide dissolves in water to form an acidic solution. A non-metal oxide dissolves in water to form an acidic solution. NOT SURE If we keep diluting an acid solution it will eventually turn alkali. Ca+OH- is the ionic formula for Calcium Hydroxide. Find the volume of solution if 0.8 mol l-1 contains 160g of Sodium Hydroxide Solid acidic compounds conduct electricity. 0.25 mole of sodium hydroxide is 10g. H+ and OH- ions are in There are more H+ ions Group 1 and 2 metal equal quantities in pure in an alkaline solution hydroxides are soluble. water. than OH- ions To find a pH of a solution we would use wide range then narrow range pH paper Group 1 and 2 metal oxides are insoluble. 0.5 mole of H2(SO4) is 49g. There are 14.2 moles in 1kg of Ammonium Sulphate Ionic compounds are not molecules. Sulphur dioxide and nitrogen dioxide cause acid rain. Soluble Indigestion tablets make alkaline solutions The concentration of 20g of NaOH dissolved in 0.25l of water is 4 mole l-1 During electrolysis of an acid hydrogen evolves at the negative electrode.











