Solutions WS Answers Section 2
advertisement

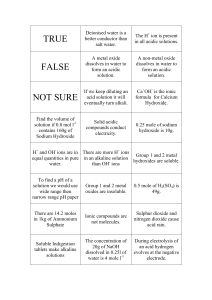
Solutions WS Answers Section 2-3 VOCABULARY REVIEW 1. A solvent is a substance in which a solute is dissolved. 2. An aqueous solution is a solution in which water is the solvent. 3. A hydroxide ion is a negatively charged ion consisting of one oxygen atom and one hydrogen atom, OH–. 4. A base is a solution that contains more hydroxide ions than hydrogen ions. 5. A buffer is a chemical substance that neutralizes small amounts of either an acid or a base added to a solution. MULTIPLE CHOICE 1. c 2. b 3. a 4. c 5. d SHORT ANSWER 1. Solutions are mixtures in which one or more substances are uniformly distributed in another substance. Solutions can be composed of liquids, solids, or gases. 2. The solution contains 10 g of sugar, and the solvent is water. 3. Acidic: more H+ ions than OH– ions. Alkaline: more OH- ions than H+ ions. Neutral: equal number of H+ and OH- ions. 4. The solution with a pH of 9 has 106, or 1,000,000, times more hydroxide ions. 5. By neutralizing small amounts of acid or base that may be added to a solution, buffers keep pH values at normal and safe levels. The control of pH is essential for the function of enzymes. 3 6. Since a tenfold increase in H+ ion concentration reflects a decrease of one pH unit, a 100-fold increase in concentration reflects a decrease of two pH units. Therefore, the new pH would be 5.5. STRUCTURES AND FUNCTIONS a, alkaline; b, neutral; c, acidic