What You Need to Know About Acids and Bases
advertisement

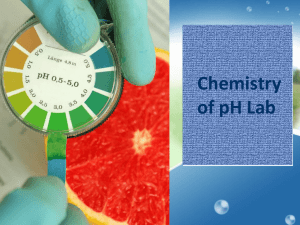
Pg. 77-78 of composition notebook Acid: Substance that donates Hydrogen ions (H+) to a solution. Or forms H3O+ (hydronium ions) with water Base: Substance that donates hydroxide ions (OH-) to a solution. H2O Water H+ hydrogen ion + OH- + hydroxide ion pH is a way to measure the amount of acid in solution Scale ranges from 0 to 14 A pH of 7 = neutral (neither acidic nor basic) pH below 7 is acidic pH greater than 7 is basic Examples: Acetic acid (vinegar), carbonic acid (carbonated beverages), hydrochloric acid (stomach acid), lemon juice Have a sour taste React with metals Produce color changes in indicators Litmus paper-turns blue litmus paper red Examples: Aluminum Hydroxide (deodorant), antacids, sodium hydroxide (soap production, drain cleaner) Bitter taste Slippery feel (think soap) Color changes in indicators Red litmus paper will turn blue Phenolphthalein will turn red 1. 2. 3. What does red and blue litmus paper tell you about a substance? Do you need to use both pieces of litmus paper to sample a substance? Why or why not? What would it tell you about a substance if neither paper changed color? Question: Which foods and household substances are acids and which are bases? Data: Substance 1-Tap Water 2-Lemon Juice 3-Vinegar 4-Cola 5-Mouth Wash 6-Antacid 7-Coffee 8-Milk 9- Baking Soda pH Prediction Actual pH Substance Tap water Liver homogenate Initial pH pH with 1 drop of acid pH with 2 drops of acid pH with 3 drops of acid WITHOUT looking at your notes (or book, or discussing with another person), write down what you remember about acids and bases. For each substance discuss the following in complete sentences: Share the name and measured pH value of the substance. Explain how the pH of the substance influences its properties, uses and side effects (or lack thereof). Based upon your results in part B, what do you think a function of the liver is? Use your specific data to support your answer. Why did we add the acid to water as well?