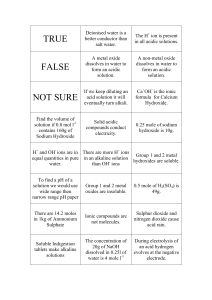
Q- Give some characteristic properties of acids and bases Ans- some characteristic properties of acids and bases Sr.No. 1 2 3 4 5 Property Taste Effect on blue litmus Effect on red litmus Effect on skin Electrical conductivity Acid Sour Turns red No effect Corrosive Aqueous solutions conduct electricity Base Bitter No effect Turns blue Harm skin tissue Aqueous solutions conduct electricity Q4-Give name ,formula and uses of four Common Acids AnsName Hydrochloric acid Nitric acid Sulphuric acid Phosphoric acid Formula HCl HNO3 H2SO4 H3PO4 Common use Cleaning of metals, bricks and removing scale from boilers Manufacture of fertilizers, explosives Manufacture of many chemicals, drugs, dyes, paints and explosives. Manufacture of fertilizers, acidulant for food Q5-Give name ,formula and uses of four Common bases AnsName Formula Common use Sodium hydroxide Potassium hydroxide Calcium hydroxide Magnesium hydroxide NaOH KOH Ca (OH)2 Mg(OH)2 Soap making, drain cleaners Making liquid soap, shaving cream Making mortar, plasters, cement Antacid, laxative Q- What is The pH scale? Ans- The pH scale Chemists use a number scale from 0 to 14 to describe the concentration of H + ions in a solution. It is known as pH scale. Explanation i-A pH of 7 indicates a neutral solution. ii-Acids have pH less than 7. ii-Bases have pH greater than 7. The pH scale In 1909, the Danish biochemist Soren Sorenson proposed a convenient method to express such a small concentration of H+ ions and OH- ions by pH or pOH pH is defined as the negative logarithm of the molar concentration of H+ ions in aqueous solutions. pH = -log [H+] For pure water at 25OC [H+] = 1x10-7M, [OH-]=1X10-7M pH= -log (1x10-7) = 7 ThuspH of water is 7. All aqueous solutions with pH= 7 at 25oCare neutral. If pH is less than 7, the solutions become acidic, [H+] increases and [OH-] decreases. pH range and Type of solution When [H+] = [OH-] = 1 x 10-7, solution is neutral When When [H+] >1 x 10-7, solution is acidic [H+] < 1 x 10-7, solution is basic Methods of measurement of pH Scientist use different methods to measure pH of a solution. pH paper or universal indicator paper is used to measure pH of a solution. Methods 1-pH paper pH paper is dipped in the solution. The colourthat develops on the pH paper is compared to the colour corresponding to a known pH on the chart. Each colour is linked to a specific pH value. Colours of pH paper or universal indicator 2-pH Meters: There are machines called pH meters to measure pH .This instrument is dipped into solution of unknown pH.It shows pH of the solution on digital screen.If pH is lower than 7 it is an acid and if it is more than 7 it is a base. Q- Give some applications of pHSirShahid.M 92733715 Ans-- Analytical chemist measures pH of solutions pH measurement has valuable applications. For instance it helps analytical chemist to (i) to create soil conditions ideal for plant growth (ii) medical diagnose (iii) maintaining the correct acid base balance in swimming pools (iv) (iv) electroplating (v) (v)manufacture of medicine etc. tap water and waste water.