pH Scale, Acids, and Bases Explained
advertisement
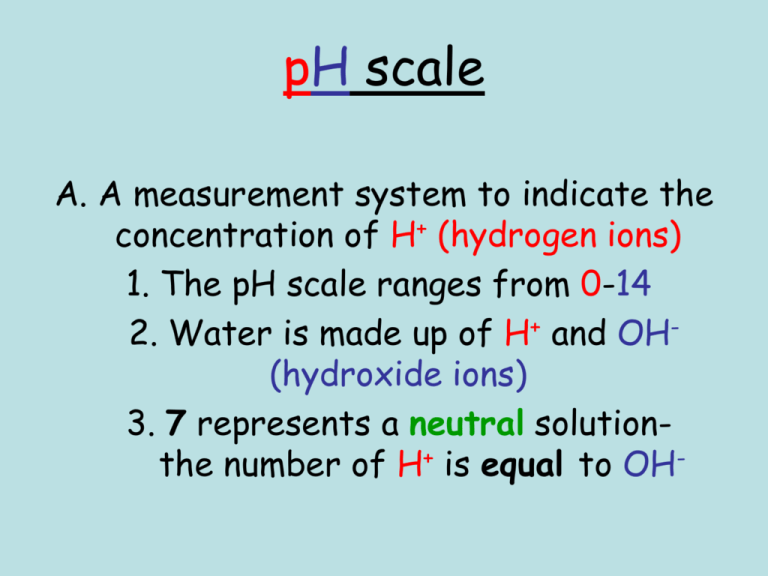
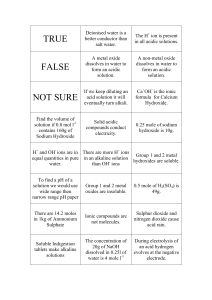
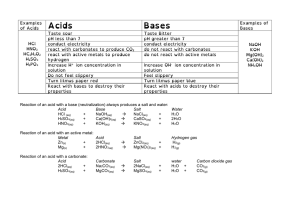
pH scale A. A measurement system to indicate the concentration of H+ (hydrogen ions) 1. The pH scale ranges from 0-14 2. Water is made up of H+ and OH(hydroxide ions) 3. 7 represents a neutral solutionthe number of H+ is equal to OH- B. An acidic solution (acid) has more H+ than OH-. 1. An acidic solution would be anything less than 7 the pH scale (further from neutral the stronger the acid) 2. An acidic solution would range in color from yellow to red on pH paper 3. An acidic solution has a sour taste 4. Examples of acids Acetic acid (vinegar) Citric acid ( lemons) Stomach acid ( HCl- hydrochloric acid) (very strong acid) C. A basic solution (base) would have more OH- than H+. 1. A basic solution is anything greater than 7 on the pH scale ( the further from 7 the stronger the base) 2. A basic solution ranges from green to dark blue on pH paper 3. A basic solution has a bitter taste and a slippery feel 4. Examples of bases 1. Ammonia (NaOH) (very strong base) 2.Bleach 3. Blood • What would happen if you mix a very strong acid with a very strong base? HCl + NaOH+ What is NaCl? What is H2O? HO NaCl ____ +_____ 2 The pH Scale N E U T R A L ACID 0 1 RED 2 3 4 5 ORANGE 6 7 BASE 8 YELLOW 9 10 11 12 13 14 LIGHT BLUE DARK BLUE vinegar ammonia Question #1 • Lemon juice has a pH of 2.2. Is it an acid or a base? Question #2 • Detergents such as Tide have a pH of about 10. Is Tide an acid or a base? Question #3 • Seawater has a pH of 8.2. Is seawater an acid or a base? Question #4 • The inside of your mouth has a pH of 7. Is it an acid or a base? Question #5 • How do you think we came up with the term “acid rain”?