PreLab Questions: Dehydration Please type and submit the answers
advertisement

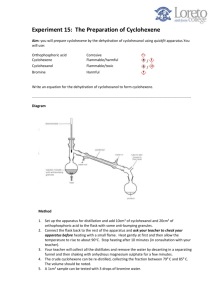
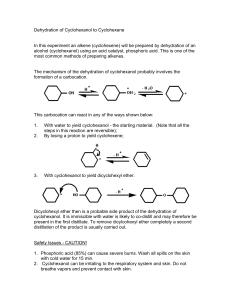
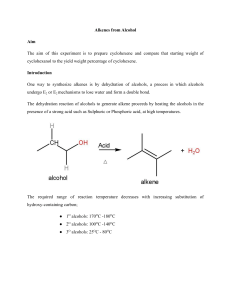
PreLab Questions: Dehydration Please type and submit the answers to the following questions: 1. The 100 mL reaction flask for this reaction should be clean but does not have to be dry before starting. Why? 2. What apparatus is employed to drive the equilibrium of this reaction towards product formation? 3. After the distillation, the product is “purified” in a process generally referred to as “the work-up”. Certain reagents were added to your reaction solution to remove unwanted components. What is removed by each of the following? a. aqueous sodium hydroxide solution b. calcium chloride 4. What’s the difference between washing and extracting? 5. Why must you remove the stopper from the separatory funnel, before draining the bottom layer out? For Questions 6-9, consider the following scenario: Run a reaction using 9 mL of cyclohexanol and 7.0 mL of 9 M sulfuric acid in order to form cyclohexene. Show work for conversions. 6. In this experiment, sulfuric acid is not to be considered in the calculations to determine the limiting reagent. Why? 7. Calculate the number of moles of cyclohexanol used in this scenario. 8. What would be the theoretical yield (in grams) of cyclohexene in this scenario? 9. If 4.33 g of cyclohexene was obtained from this scenario, what would be the percent yield?