Cyclohexene Synthesis: Dehydration of Cyclohexanol Lab
Dehydration of Cyclohexanol to Cyclohexene
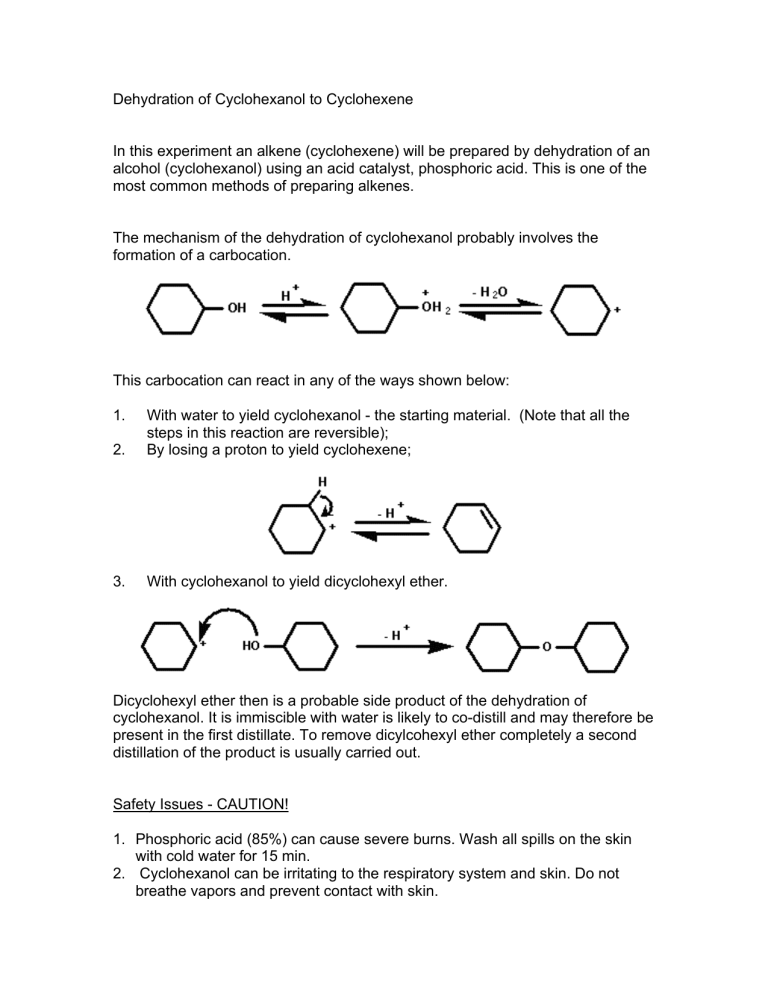
In this experiment an alkene (cyclohexene) will be prepared by dehydration of an alcohol (cyclohexanol) using an acid catalyst, phosphoric acid. This is one of the most common methods of preparing alkenes.
The mechanism of the dehydration of cyclohexanol probably involves the formation of a carbocation.
This carbocation can react in any of the ways shown below:
1. With water to yield cyclohexanol - the starting material. (Note that all the steps in this reaction are reversible);
2. By losing a proton to yield cyclohexene;
3. With cyclohexanol to yield dicyclohexyl ether.
Dicyclohexyl ether then is a probable side product of the dehydration of cyclohexanol. It is immiscible with water is likely to co-distill and may therefore be present in the first distillate. To remove dicylcohexyl ether completely a second distillation of the product is usually carried out.
Safety Issues - CAUTION!
1. Phosphoric acid (85%) can cause severe burns. Wash all spills on the skin with cold water for 15 min.
2. Cyclohexanol can be irritating to the respiratory system and skin. Do not breathe vapors and prevent contact with skin.
3. Cyclohexene has an unpleasant smell. Cover all containers of this compound and do not leave drying agent, glass wool or towels coated with the compound lying on the bench.
4. Bromine causes severe burns. Keep bromine away from your skin. Do not breathe the vapors. Use only in the fume hood.
Procedure:
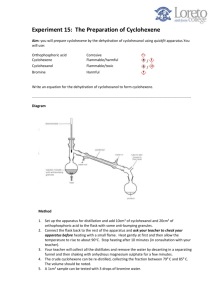
Assemble a simple distillation apparatus, using the 50 mL round-bottom flask.
The thermometer should be set lower than usual so that the bulb is in the ground glass area rather than at the junction of the adaptor. Use a large test tube as the receiver; the test tube should rest in a beaker filled with ice.
Pour 10 ml of cyclohexanol into a 10 ml graduated cylinder and weigh the full graduated cylinder. Pour the alcohol into the round bottom flask of the distillation apparatus. Now reweigh the empty graduated cylinder to determine the mass of alcohol you used.
Be very careful here!
Add 3 mL of 85% phosphoric acid to the alcohol in the round-bottom flask and swirl to mix. Add a boiling stone and reconnect the distillation apparatus. Turn on the heating mantle to start heating this mixture slowly (make sure the water is flowing through the condenser). Gradually increase the voltage to the mantle to increase the temperature. You will probably need to insulate the flask (make an aluminum foil shield). This process of reaction and distillation will work best if it takes 45 minutes or more with slow heating. Distill the products until the production of distillate is negligible, making sure the thermometer stays below 110 o C. Some residue will remain in the reaction flask; do not boil it dry.
Turn off the power and remove the heating mantle. About 1 to 2 ml of a brown liquid - very bad stuff - will remain when you have finished the reaction. This needs to be cooled before you add cold water to it for cleaning purposes.
The crude product is contaminated with water, unreacted alcohol, phosphoric acid and side product dicyclohexyl ether. We will only worry about removing the water. Water in the distillation receiver should appear as a separate phase
(more or less dense than cyclohexene?). Carefully decant the product into a clean, dry test tube. You should try to transfer as much cyclohexene as you can, while attempting to leave any water behind. If a large amount of water is transferred, you may need to repeat this step. Once you have no visible water, add a small amount of calcium chloride solid to dry your product completely.
( Note : you may be instructed to use a different drying agent.) Wait 15 minutes.
Weigh a clean, dry test tube. Decant the dry product to the test tube and weigh it again. Record your yield. Show the instructor your product, and then add it to
the pooled sample he will show you.
Identification test: Work in the hood!
You should carry out the following test to confirm the presence of alkenes.
Place a counted number of drops (3-5 should be enough) of cyclohexanol or your product in a small test tube. Add a solution of bromine in chloroform dropwise until the bromine color persists. How many drops are required for each sample?
Clean-up : Clean the cooled reaction flask and adapter with acetone and put the washings in the waste bottle in the hood. DO NOT wash the flask in the sink.
Post-Lab: Report the following information in your notebook
♦
weight of the cyclohexene product;
♦
percent yield;
♦
boiling range of your product;
♦ Differences in physical characteristics (color, odor, etc.) between starting materials and product;
♦
Write the reactions and structures of products formed in the chemical identification test.
Rev 9/2009 Steve Bowlus