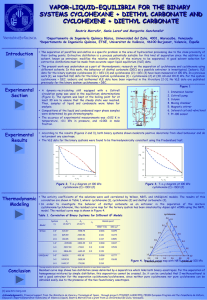
EXPERIMENT 1: ALKANES & ALKENES: COMBUSTION AND ACTION OF SULPHURIC ACID Introduction: Hydrocarbon is any organic chemical compound composed only of the elements carbon (C) and hydrogen (H). If a hydrocarbon contains only single bonds, it is an alkane. If it contains one or more carbon-carbon double bonds, it is classified as an alkene. If it contains one or more triple bonds between two carbon atoms, it is an alkyne. Alkanes are described as saturated hydrocarbons while alkenes and alkynes are said to be unsaturated. Alkanes are linear, or branching, compounds that are made of varying numbers of carbon atoms that are all saturated with hydrogen atoms. The formula for an alkane is C nH(2n+2). Alkanes are relatively unreactive and not often involved in chemical reactions. The carbon in alkane is sp3 hybridized forming a tetrahedral complex with bond angles of 109.5°. Each C—C and C—H bond is a sigma (σ) bond. Alkenes are like alkanes, but they have at least one double bond between carbon atoms and the number of double bonds may vary. The double bond consists on one sigma (σ) bond and one pie (𝜋) bond. An alkene with only one double bond has the formula CnH2n which is two hydrogen atoms less than alkanes. Alkenes are more reactive than alkanes due to the presence of double bonds. Different from alkane, the carbon in alkene is sp2 hybridized. All hydrocarbons will burn in the presence of oxygen (in the air). This reaction is called combustion and the products of this reaction are carbon soot, carbon dioxide and water. Combustion of equal amounts of different hydrocarbons will yield different quantities of carbon dioxide depending on the ratio of carbon to hydrogen in molecules of each .Here is an example of a combustion reaction: C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (g) + heat Cyclohexane is an alkane while cyclohexene is an alkene. They both have a ring structure but cyclohexene contains double bond. In this experiment, we will be burning cyclohexane and cyclohexene. The flame produced by cyclohexane will be clean while the flame for cyclohexene will be sooty due to the difference of number of hydrogen atoms per molecule content. Next, we will add both cyclohexane and cyclohexene to sulphuric acid. Cyclohexane is a saturated 1 compound however cyclohexene is more reactive. Hence, cyclohexane will react with sulphuric acid not as rapidly as cyclohexene. Objectives: 1. To make careful observations and compare the differences(s) between alkanes and alkenes. Chemicals/Apparatus: Cyclohexane, Cyclohexene, 1M of sulphuric acid, test tubes and racks, watch glass, matches Methods: A) Combustion (to be performed under fume hood) 1. 2-3 drops of liquid cyclohexane are dropped onto a watch glass. 2. Fire is set to it. 3. The flame is observed (whether it was clean or sooty). 4. Steps 1 - 3 are repeated with cyclohexene. B) Action of sulphuric acid (to be performed under fume hood) 1. 1mL of 1M concentrated sulphuric acid is transferred in a dry test tube held in a rack carefully. 2. 0.5 mL of the liquid cyclohexane is added. The test tube is shook gently. 3. Any changes are observed. 4. 1mL concentrated sulfuric acid is put in a dry test tube held in a rack. 5. Steps 1 - 4 are repeated with cyclohexene. 2 Results: Experiment Observation(s) Cyclohexane Cyclohexene Combustion Clean Sooty Action of sulphuric acid Two separate layers of The solution mixed to form a precipitate was formed blackish brown colour Question: A) Combustion (to be performed under fume hood) 1. Is the flame clean or sooty for combustion of cyclohexane? (A clean flame is necessary for a substance to be used as a fuel). What does the flame indicate about the ratio of C:H? For cyclohexane, the flame is clean due to lower percentage of carbon compared to cyclohexene. Hence, cyclohexane has a lower C:H ratio. 2. What are products of combustion of alkenes? How do they account for the sooty and luminous flame? Products of combustion of alkenes are carbon dioxide and water. Alkenes burn with a sooty and luminous flame because they have a higher percentage of carbon per molecule and so do not completely oxidize in the air. As soot is basically unburnt carbon, therefore, due to incomplete combustion in air, the flame is sooty. B) Action of sulphuric acid (to be performed under fume hood) 1. When concentrated sulphuric acid was added into cyclohexane, is there any sign of reaction? No, there was no reaction. The cyclohexane remained colourless. 2. Do the substances mix or are there two separate layers in the test tube when concentrated sulphuric acid was added into cyclohexene. Explain the reason behind the reaction. There were two separate layers in the test tube when sulphuric acid is added into cyclohexene. Cyclohexene contains double bond making it unsaturated and thus 3 undergoes electrophilic addition with sulphuric acid. Cyclohexene’s double bond will break down into single bond. The new bond will connect to a hydroxyl group. Hence, a two layer precipitate will be formed. Discussion: Cyclohexane and cyclohexene are hydrocarbons that consist of only carbon and hydrogen atoms. Moreover, we can classify them as saturated or unsaturated hydrocarbon. Cyclohexane is a cyclic molecule with the formula of C6H12. This is a cycloalkane. Cyclohexane is a saturated hydrocarbon as there are no double bonds or triple bonds between carbon atoms. Since this is a cycloalkane, it less reactive comparatively. On the other hand, cyclohexene is a cycloalkene with the formula of C6H10. It is nearly similar to cyclohexane, but there is one double bond between two carbon atoms in the ring, which makes it an unsaturated hydrocarbon. Since cyclohexene has a double bond, it can undergo reactions that are characteristic of alkenes. 4 During combustion test, both cyclohexane and cyclohexene produced carbon dioxide and water. However, cyclohexene burnt and produced more soot because of the higher percentage of carbon compared to cyclohexane – they have a higher C:H ratio. Hence, it can be concluded that alkanes produce less colour intensity and less soot was given off during combustion test compared to alkene. The chemical equation for the combustion of cyclohexane is: C6H12 (l) + 9O2 (g) 6CO2 (g) + 6H2O (l) The chemical equation for the combustion of cyclohexene is: 2 C6H10 (l) + 17 O2 (g) 12 CO2 (g) + 10 H2O (g) When sulphuric acid was added to cyclohexene, cyclohexene reacted by addition, forming alkyl hydrogen sulphate. The 𝜋- electrons of alkenes are very reactive towards sulphuric acid. The 𝜋- eleectrons attack the H+ ions to form a carbocation which reacts with the bisulphate anion. The colour change is a characteristic that a reaction occurred. As a result, the solution turned cloudy and heat was released due to the breaking down of cyclohexene’s carbon - carbon double bond. However, for cyclohexane, it was seen that cyclohexane is inert to concentrated sulphuric acid. Hence, no visible change occurred and there were two layers of precipitate formed: cyclohexane on top and sulphuric acid at the bottom. Conclusion: In this experiment, the differences between alkanes (cyclohexane) and alkenes (cyclohexene) are identified. It can be seen that alkenes are more reactive than alkanes due to the presence of double bonds. Alkenes showed a lot of reactions such as combustion and addition while alkane only showed combustion. 5 References: 1. Energyeducation.ca. (2018). Hydrocarbon combustion - Energy Education. [online] Available at: https://energyeducation.ca/encyclopedia/Hydrocarbon_combustion. [Accessed 28 Jan. 2020]. 2. Coursehero.com. (2020). [online] Available at: https://www.coursehero.com/file/32405017/Experiment-1-Alkanes-alkenes-Combustionand-action-of-sulphuric-acid/. [Accessed 28 Jan. 2020]. 3. Experiment 5 -Reactions of Hydrocarbons. (n.d.). [online] Available at: https://laney.edu/cheli-fossum/wp-content/uploads/sites/210/2012/01/5-Reactions-ofHydrocarbons.pdf. [Accessed 28 Jan. 2020]. 4. Chemistry LibreTexts. (2016). 22.2: Alkanes, Cycloalkanes, Alkenes, Alkynes, and Aromatics. [online] Available at: https://chem.libretexts.org/Courses/Heartland_Community_College/HCC%3A_Chem_16 2/22%3A_An_Introduction_to_Organic_Chemistry/22.2%3A_Alkanes%2C_Cycloalkan es%2C_Alkenes%2C_Alkynes%2C_and_Aromatics. [Accessed 28 Jan. 2020]. 5. Carey, F.A. (2018). hydrocarbon | Definition, Types, & Facts. In: Encyclopædia Britannica. [online] Available at: https://www.britannica.com/science/hydrocarbon. [Accessed 28 Jan. 2020]. 6. www.onlinemathlearning.com. (2018). Alkane, Alkene Tests (examples, answers, activities, experiment, videos). [online] Available at: https://www.onlinemathlearning.com/alkane-alkene-tests.html. [Accessed 28 Jan. 2020]. 7. Dunee (2012). Difference Between Cyclohexane and Cyclohexene. [online] Compare the Difference Between Similar Terms. Available at: https://www.differencebetween.com/difference-between-cyclohexane-and-vscyclohexene/ [Accessed 28 Jan. 2020]. 8. Chemguide.co.uk. (2020). electrophilic addition - symmetrical alkenes and sulphuric acid. [online] Available at: https://www.chemguide.co.uk/mechanisms/eladd/symh2so4.html [Accessed 29 Jan. 2020]. 6