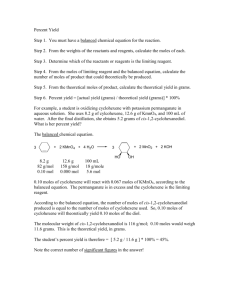
DEPARTMENT OF PURE AND APPLIED CHEMISTRY Visayas State University, Baybay, Leyte CHEM126 Organic Chemistry II Laboratory Report Name Course/Yr Group No : Mark Ryan R. Tripole : BS Chemistry II : 2 Date Performed Date Submitted Score : 11/27/2014 : 12/09/2014 Experiment No. 2 Preparation of Cyclohexene I. Introduction Looking at it from the perspective of study, alkene chemistry is one which is quite broad because of the many reactions that the particular class of organic compound undergoes. The reactions are always reversible in the sense that if one were to try to convert a particular alkene into, let’s say, an alcohol through the process of hydrolysis, then the reverse could be achieved by the dehydration of the alcohol back into the parent alkene. The main objective of this experiment was to observe the laboratory procedure for the synthesis of cyclohexene from cyclohexanol, which, as already mentioned before, is though the dehydration of the parent alcohol to introduce the unsaturation. This dehydration, or removal of water, is characteristic of a class of reactions known as Elimination reactions. The classification and idea is somewhat the same as that of Substitution reactions, but the key difference lies in the fact that in elimination reactions, connections are being broken and those molecules that have broken away are completely removed or “eliminated”. This introduces the other objective, which was to gain a functional understanding of these Elimination reactions and the mechanisms that makes them happen. Though there are two main classifications of Elimination reactions, namely the E1 and the E2, there will be emphasis only on the E1 because that is the main mechanism through which the dehydration of the cyclohexanol actually takes place. Further elaboration on the respective mechanisms will be shown in the succeeding sections. II. Results The experimental procedure was all about the synthesis of cyclohexene from the dehydration of cyclohexanol. Much like the previous experiment, there was emphasis on the accuracy of the overall procedure so as to obtain the maximum amount of end product possible. Tabulated and shown below are the results obtained from the procedure, including any relevant calculations that were made with respect to the data. Further elaboration on the results will be in the Discussion section of the laboratory report. Page 1 of 13 Calculations relative to the experiment (in reference to tabulated values) Taking into consideration that cyclohexanol is the only reactant, thus it is the limiting reagent. It is already known that 20.0g of cyclohexanol was used in the procedure. Using this information and the molecular weights of the cyclohexanol and the cyclohexene, the theoretical number of moles of the cyclohexene that can be obtained can be calculated: Molecular weight of cyclohexanol 100g/mol Molecular weight of cyclohexene 82g/mol No. of moles of C 6 H10 obtainable 20.0g C 6 H11OH x 1 mol C 6 H11OH 1 mol C 6 H10 x 100g C 6 H11OH 1 mol C 6 H11OH 0.20 mol C 6 H10 Now that it is known that 0.20 moles of C6H10 can be obtained, it’s all a matter of converting it to grams by multiplying the number of moles to the molecular weight of cyclohexene to obtain the theoretical yield: Theoretical yeild of C 6 H10 0.20 mol C 6 H10 x 82g C 6 H10 1 mol C 6 H10 16.4 g C 6 H10 Page 2 of 13 Basing on the data from the experiment, we had only obtained 8 grams of final product, and in comparison with the theoretical yield, the percentage yield can be calculated as follows: actual yield (g) x 100% theoretical yield (g) Plugging in the related values Percentage yield 8 grams x 100% 16.4 grams % yield 48.8% % yield Tabulated below are the results obtained from the Bromine test and the Baeyer’s test on the final product. The field for the Bromine test is left blank because the procedure was not conducted due to the danger in handling such chemicals at the present time. III. Discussion This experiment was all about observing the laboratory procedure in the preparation of cyclohexene from cyclohexanol. As has already been established in the introductory portion of this laboratory report, this conversion comes about from the catalytic dehydration of the alcohol to introduce the unsaturation into the structure. As a preliminary, this dehydration falls under the mechanisms of Elimination reactions, specifically one of the E1 (Unimolecular Elimination) nature. Basically speaking, the E1 mechanism is similar in pattern with the SN1 mechanism and in the sense that it is also a two step process. Though they are fundamentally different in terms of their fast second step, the two mechanisms share the same first rate determining step, which is the loss of a leaving group and the formation of a carbocation. To illustrate the E1 pathway further, below is a simple schematic diagram for a simple E1 reaction: Page 3 of 13 Similar to the SN1 mechanism, reactivity for the E1 pathway follows the stability of the carbocation intermediate. The more stable the carbocation intermediate, then the more favored they are in the E1 mechanism (in short, 3° > 2° > 1°). And in retrospect, we also see the dependence of the efficiency of the E1 pathway (similar to the SN1 mechanism) on the leaving group. The better the leaving group, then the easier it is for the E1 mechanism to occur. For the purposes of this laboratory report, let us take an alcohol as an example. An alcohol is an organic compound that possesses the hydroxyl or –OH functional group. By definition, the –OH group is a terrible leaving group, so first it has to be protonated in order to convert it into a better leaving group. In relation to the E1 mechanism, if the substrate is an alcohol, then a strong acid will be required to protonate the OH portion. In order to look deeper into the reaction mechanism, the simple conversion of cyclohexanol to cyclohexene was observed in the experiment. As already mentioned, since the hydroxyl group on the cyclohexanol is a really bad leaving group, then it has to be protonated in order to convert it into a good leaving group. The acidic condition to favor this was provided through the usage of 85% phosphoric acid. To move on with the discussion, below is a simple sketch for the experimental procedure conducted: Page 4 of 13 Moving on to the mechanisms, when an alcohol like cyclohexanol is placed in acidic conditions such as that provided by the 85% phosphoric acid, the –OH functional group on the cyclohexanol is protonated: Now that the OH group has been protonated, it has been converted from a bad leaving group into a better one. The positive charge on the oxygen is something that it doesn’t want, so it will break away from the carbon it is attached to (hereafter referred to as the α-carbon) to neutralize itself. This loss of the water leaving group facilitates the formation of a carbocation: At this point, it is important to take note that the SN1 and E1 pathways are competing with each other. If the SN1 mechanism were allowed to occur, then the final yield for the desired cyclohexene will be less than desirable. One of the purposes of the phosphoric acid is to suppress the SN1 pathway, because it provides conditions favorable for the E1 mechanism and it has an anion that is a relatively poor nucleophile. Now that the SN1 pathway is out of the way, the water present in the solution acts as a base and abstracts a hydrogen from the neighboring β-carbons: Page 5 of 13 It is imperative to realize that the abstraction of the hydrogen can occur at any two of the β-positions, but ultimately in the end the product is still the same. There is usually selectivity in terms of from which β-carbon the hydrogen will be abstracted, but since the molecule described is symmetrical in nature, it doesn’t really matter as of now. The resulting hydronium at the end is significant of the regeneration of the catalyst as used in the initial protonation step. This whole reaction is basically achieved up until the first distillation portion, but the by-products from the side reactions and eventual work up have to be considered as well. Considering the by-products that might arise from the reaction, one has to take note that most if not all of the steps are pretty much reversible. So upon formation of the carbocation, there are a few possibilities as to what could happen. First, the carbocation could react with the water (by virtue of its nucleophilic tendencies) again to reform the initial charged substrate and then initial cyclohexanol: Then of course, from there we have the reaction yielding the desired cyclohexene which has already been shown and discussed in the previous page. Another possible byproduct comes from the consideration that since the cyclohexanol molecule possesses the hydroxyl functional group, it has partial charges. The partial negative charge on the oxygen is of note in this scenario because it allows the molecule to act as a weak nucleophile. Thus a cyclohexanol molecule could react with the formed carbocation to form dicyclohexyl ether as shown below: As a work up, standard techniques have been employed in order to obtain the purest possible product and the maximum amount of yield. After the initial distillation, the solution was first saturated with solid sodium chloride. This was a pre-drying step that aided in removing most of the water present in the distillate. The principle behind the mechanism of the solid sodium chloride is that it pulls water from the organic layer (which contains the cyclohexene) into the water layer. This is connected to the principle that the resulting concentrated salt solution formed by the salt with the aqueous layer wants to become more dilute and also because salts have a stronger attraction to water than to organic solvents. Another way to look at this could Page 6 of 13 also be the “salting out” of the organic material from the aqueous layer. So saturating with the sodium chloride serves the dual purpose of pulling out water from the organic layer and expelling any organic material from the aqueous layer, all in the context of “like dissolves like”. The dissolving of sodium chloride in the water and the presence of ions increases the ionic strength of the aqueous layer. Since cyclohexene in itself is a relatively non-polar compound, it does not dissolve the sodium chloride. A simple diagram illustrating this concept is shown below: After this step, an amount of sodium carbonate was added to the solution, just enough to make it basic to red litmus paper. This step ensured the removal of any left over phosphoric acid through the following reaction: 2H3PO4 + 3Na2CO3 2Na3PO4 + 3CO2 + 3H2O The resulting sodium phosphate compound is soluble in the aqueous layer, and this was easily removed along with the aqueous layer using a separatory funnel. The final work up step before the final distillation was the final drying step where anhydrous calcium chloride was added to the crude cyclohexene. The hygroscopic nature of the calcium chloride aided in the final removal of any water that could have possibly been left over. After separating, the second distillation was then performed, and it is at this stage where any dicyclohexyl ether formed is separated from the cyclohexene. The boiling point of cyclohexene is around the 80 to 85°C mark, while the dicyclohexyl ether clocks in at around the 242.5°C mark. Any liquid left over in the distilling flask that didn’t really boil over as a distillate was the diclohexyl ether. DISCUSSION ON YIELD With regard to the yield, the group had obtained only 48.8% of product, which is a far shot from the expected 79-80% that was to be expected from the proportions of the chemical species. One of the main reasons for this low output may have been due to human error. Error in accurately measuring the amount of initial reactants, error at the separation stage where some of the organic layer might have actually escaped as well as not having set up the distillation apparatus correctly, wherein some of the gaseous cyclohexene might have escaped out of the apparatus. The group had encountered this a few times through the experiment before the connections were adequately sealed and the “fuel-like” odor of cyclohexene was no longer observed. Page 7 of 13 UNSATURATION TESTS For the purposes of this experiment, tests had to be carried out in order to determine whether the final distillate was actually the cyclohexene. Thus tests for unsaturation were to be carried out on the final product to test for the double bond functionality. Two tests of note will be mentioned here, the first being the Bromine test (using Br2 in dichloromethane) and the Baeyer test (using KMnO4). Because of the danger in the handling of bromine as of the time, the bromine test was not carried out, but the mechanism and the expected result will be discussed here. Bromine in Dichloromethane The reaction between bromine and an alkene is characteristic of an addition reaction, where the bromine “adds” across the double bond to form a dihalide. In its original form, the bromine solution is reddish or quite red in color. On reaction with an alkene, the alkene loses unsaturation and takes in the Bromine, and what happens is that there is a gradual disappearance of the red color of the solution. This is the positive indication that the unknown compound indeed has unsaturation and is an alkene or an alkyne. The addition of Bromine across a double bond is characteristic of a mechanism known as Electrophilic Addition. A step by step illustration of this process is shown below with respect to cyclohexene: STEP 1 The loosely held π electrons of cyclohexene induce a dipole in bromine. One of the bromine leaves as an anion and the other gets connected to the ring. STEP 2 The nucleophilic the carbocation compound. The species is known ion. bromine attacks portion of the resulting ionic as a bromonium STEP 3 A bromide ion then goes on to attack the bromonium ion, and this results in a dihalide. The reddish color of the bromine solution disappears as it is used up. The 1,2-dibromocyclohexane formed is not of prime importance in this test, because it is the physical disappearance of the color that is the major result. The dichloromethane solvent only serves the purpose of dissolving the bromine and does not play a part in the reaction whatsoever. In the succeeding portion will be a discussion on the other unsaturation test used in the experiment, the Baeyer’s test which employs the usage of dilute Potassium Permanganate solution (KMnO4) Page 8 of 13 Baeyer’s Test (Dilute Potassium Permanganate KMnO4) The use of Potassium Permanganate solution is also one of the more functional tests for unsaturation. Compared to the bromine test, this one is much safer and the results are just as immediate. The original color of potassium permanganate in its solution form is a dark purple. On reaction with an unknown that is suspected to have unsaturation, the purple coloration of the solution is gradually replaced by a brownish solution that contains a precipitate of the same color. In terms of what happens to the original compound, the alkene is converted into a diol in this process. An illustration of the mechanism behind this reaction with KMnO4 is shown below: First the permanganate ion approaches the alkene and basically complexes it forming the product as shown. Then through a reaction with water (whose mechanism would be too long and too exhaustive for the simple purposes of this laboratory report), produces the 1,2cyclohexanediol and the compound Manganese Oxide (MnO2) which is basically a brown precipitate: Relating it to the experimental results, when the final cyclohexene was tested with the potassium permanganate, there was gradual but immediate change from the purple to the brown solution. Though the brown coloration didn’t really cover the whole solution per se, the change in itself is already a significant enough result that the final product contains unsaturation. IV. Conclusion Alkene chemistry is one that is broad and demands the full faculties of attention in order for it to be understood. It provides a simple platform for learning where one can understand the machinations behind the mechanisms and how they could possibly apply to the more complex reactions. Through the study of the conversion of cyclohexanol to cyclohexene, a deeper understanding of elimination reactions has been obtained. The factors that play an important part in the reaction mechanism and how certain conditions favor them (like the acidic conditions for E1 reactions for example), the dependence of the reaction on the leaving group, the number of connections on the α-carbon and a whole lot more. Though the final yield at 46.8% may not have been all that desirable to say the least, the group is slowly getting accustomed to the distillation procedures and the precautions that have to be taken in order to obtain the optimum amount of end product possible. Moreover, study of the mechanisms behind the unsaturation tests has provided an insight into the reactivity of the carbon to carbon unsaturation, be it triple or double bonds. In conclusion, this experiment has allowed for the closer scrutiny of catalytic dehydrations of alcohols and the unimolecular elimination pathway that follows it. Page 9 of 13 V. Answers to Questions and Exercises 1. What alcohol would be the most logical choice as starting material for the preparation of 1-methylcyclohexene? Write the chemical equation for the reaction. Explain why you think your choice is the most logical. The most logical choice would be to start with using 1-methylcyclohexanol. The chemical reaction for the dehydration of 1-methylcyclohexanol with 85% phosphoric acid is shown below: Using it as a starting material is the most logical choice because it is the configuration that will yield the highest proportion and percentage of the desired product. This is in terms of the abstraction of the hydrogen from the β-carbons, and the potential products that arise from them. Abstraction of hydrogen from the β-positions inside the ring gives the same product as shown (the desired product). But abstraction from the methyl group gives another product, and the determination of which of the two is the major and the minor product comes from observing the degree of substitution. The trisubstituted 1-methylcyclohexene (or known as the Zaitsev product) predominates over the disubstituted compound that forms from the abstraction of the proton from the methyl group (also known as the Hofmann product). 2. How much of the alcohol you choose in answering Question 1 would you require as starting material if you needed to prepare 25g of 1-methylcyclohexene and if your actual percentage yield were going to be 75%? Theoretical yield = 25 grams Percentage yield = 75% actual yield Percentage yield x 100% theoretical yield actual yield 75% x 100% 25 grams Page 10 of 13 75% x 25 grams 100 % actual yield 18.75 grams actual yield Given that the theoretical yield is obtained from multiplying the number of moles of the limiting reagent by the molecular weight of the product: 25 grams No. of moles C 6 H10 (CH3 )OH x molecular weight of C 6 H 9 CH3 25 grams No. of moles C 6 H10 (CH3 )OH x 96 g/mol No. of moles C 6 H10 (CH3 )OH 0.26 moles Then calculating for the amount in grams of the 1-methylcyclohexanol to be used: Amount in grams of C 6 H10 (CH3 )OH 0.26 moles C 6 H10 (CH3 )OH x 114 g/mol C 6 H10 (CH3 )OH 1 mol C 6 H10 (CH3 )OH Amount in grams of C 6 H10 (CH3 )OH 29.64 grams 3. What is the theoretical yield of 1,2-dibromocyclohexane for the reaction of 20g of cyclohexene with 20g of bromine? (Hint: Which is the limiting reactant?) First calculating to check and see which reactant is the limiting reactant: grams of cyclohexene moles of cyclohexene moles of 1,2-dibromocyclohexane No. of moles of C 6 H10 Br2 20 g C 6 H10 x 1 mol C 6 H10 1 mol C 6 H10 Br2 x 82 g C 6 H10 1 mol C 6 H10 No. of moles of C 6 H10 Br2 0.24 moles grams of Br2 moles of Br2 moles of 1,2-dibromocyclohexane No. of moles of C 6 H10 Br2 20 g Br2 x 1 mol C 6 H10 Br2 1 mol Br2 x 160 g Br2 1 mol Br2 No. of moles of C 6 H10 Br2 0.13 moles Since Bromine produces the least moles of product, it is the limiting reagent. Theoretical yield No. of moles of limiting reagent x mol. weight of product Theoretical yield No. of moles Br2 x mol. weight C 6 H10 Br2 Theoretical yield 0.13 moles Br2 x 242 g/mol C 6 H10 Br2 Theoretical yield 31.46 grams C 6 H10 Br2 Page 11 of 13 4. A reaction is carried out for which the stoichiometry is A + 2B + 3C D + 2E + F. In this reaction 0.5 mole of A, 0.75 mole of B, and 1.0 mole of C are allowed to react. The yield of E is 0.2 mole. What is the percentage yield? A + (0.5 mol) 2B + 3C (0.75 mol) (1.0 mol) D + 2E + (0.2 mol) F Checking for which reactant is the limiting reactant: A Moles of E from A 0.5 mol A x 2 mol E 1 mol A A Moles of E from A 1 mol E A B Moles of E from BA 0.75 mol B x 2 mol E 2 mol B A Moles of E from BA 0.75 mol E A C Moles of E from CA 0.75 mol C x 2 mol E 3 mol C A Moles of E from CA 0.67 mol E Since compound C provides the least number of moles for E, then it is the limiting reagent. Taking then into consideration that actual yield of E is only 0.2 moles, the percentage yield then can be calculated accordingly. Since we are only given moles, we can use the molar ratio to calculate for an estimate for the percentage yield: actual yield (mol) x 100% theoretical yield (mol) Plugging in the related values 0.2 mol % yield x 100% 0.67 mol % yield 29.85% Percentage yield Page 12 of 13 VI. References “Alkenes: Structure and Preparation via Elimination Reactions” David Klein, Organic Chemistry: Page 353 “The Elimination Mechanism” “Alcohols and Ethers” http://butane.chem.uiuc.edu/cyerkes/chem204sp06/lecture_notes/lect18c.html “Reaction Mechanism” http://web.pdx.edu/~wamserc/CH331F97/E3ans.htm “Synthesis of Cyclohexene” http://www.organicchem.org/oc2web/lab/exp/cyclo/cyclodes2.html “Potassium Permanganate” http://www.mpcfaculty.net/ron_rinehart/12A/chap6pag.htm “Percentage Yield” Raymond Chang, General Chemistry: Chapter 8.10 “Stoichiometric Calculations” Edwin D. Hughes, General Chemistry for Dummies, Excerpt Page 13 of 13