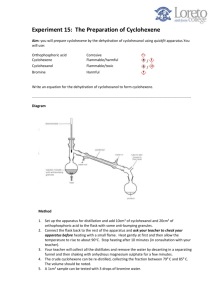
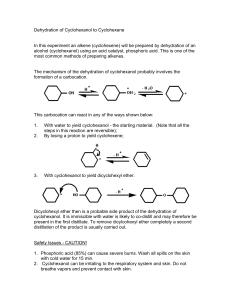
I. II. Experimental Observation After the short pre-lab lecture we had set out to gather the needed materials for the experiment Revisiting the pre-lab journal we followed the necessary steps for the experiment that we were doing It was just a matter of waiting and observing the temperature and once it hit the 45°C mark, the temperature rose at a constant speed We recorded that the first drop happened at 67°C, and the final drop happened at 82°C. The final volume was 4mL. The experiment was easy enough to follow. All of the details were jotted down on a hand out. Calculation and actual yield Weight of the test tube: 17.11 g ; weight of the test tube with the product: 19.49 g; weight of the product: 2.38 g % yield = actual/theoretical yield X 100 % = 2.38 g / 4.1 g X 100% = 58.05% Discussion and Conclusion While we were able to follow the necessary procedures that were required of us by this experiment, we cannot fully say that what we did was successful for we weren’t able to check the IR Spectrum of the final product and without this it’s really hard to tell if the experiment itself was successful. III. Post-Lab Questions 1. Why do you suppose that the dehydration of cyclohexanol to cyclohexene has been the traditional experiment to illustrate the acid-catalyzed dehydration of an alcohol to an alkene? The dehydration of cyclohexanol to cyclohexene has been the traditional experiment to illustrate the acid-catalyzed dehydration because cyclohexanol is the best starting material for it mainly for the following reasons: a.) because of its structure, it forms only one alkene upon the dehydration. b.) the rate of dehydration of cyclohexanol using 85% phosphoric acid is relatively fast c.) cyclohexene can be easily purified by distillation 2. Suppose 2-methylcyclohexanol were subjected to the conditions of this experiment instead of cyclohexanol a. What alkenes would you expect to be formed? The product that we would expect to be formed will be 1-methylcyclohexene and 3methylcyclohexene b. In what relative amounts would you expect them to be formed? 90% of 1-methylcyclohexene is expected to be formed. 3. When 3,3-dimethyl-2-butanol is boiled with 85% H3PO4 according to the procedure, an elimination reaction takes place. If a carbocation mechanism is involved and the initial carbocation undergoes elimination before rearrangement, the product should contain only 3,3-dimethyl-1-butene. If the initial carbocation should rearrange, two other alkenes could be formed: 2,3-dimethyl-1-butene and 2,3-dimethyl-3-butene. Show the mechanism that forms the three products. Predict the major and minor products. Explain your choices.