Cyclohexene Synthesis Lab Report: Dehydration of Cyclohexanol
advertisement

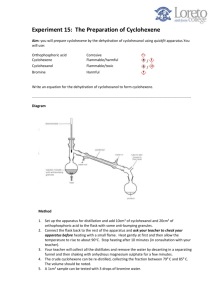
Using a Dehydration Reaction to Convert an Alcohol to an Alkene Date Due: Date Submitted: Purpose: The purpose of this experiment is to synthesize cyclohexene from cyclohexanol involving distillation and liquid/liquid separation. Also, a bromine test will be used to verify the product along with an IR spectroscopy. Chemical Structures/Reactions: + H2SO4 ⇔ + H2O Calculations: Limiting Reagent: 1 mol Cyclohexanol: 0.962 g/mL x 10mL x 100.2g x 11 mol mol = 0.0960 mol of cyclohexene 1 mol Theoretical yield cyclohexene : 0.962 x 10mL x 100.2g Percent Yield: 2.9932g 7.88g x 82.1g 1 mol = 7.88 g cyclohexene x 100= 37.98% Results: Substance Cyclohexanol Physical Description of Substance Mass of Initial Reagents (g) Mass of Substance Isolated (g) Colorless Gel-Like Liquid 9.62 N/A Percent Yield (%) N/A Distillation Temperature Range for Substance Isolated (°C) N/A Br2 Test for Substance Isolated N/A Cyclohexene Colorless Liquid 0.00 2.9932 37.98 74-80 Solution remained clear and colorless IR Spectra Table: Compound Type of Vibrational Motion Characteristic Functional Group Frequencies (cm-1) Actual Peak Location from Data (cm-1) Cyclohexanol ● ● O-H Stretch C-O Stretch ● ● 3500-3200 13200-1000 ● ● 3325.30 1064.79 Cyclohexene ● ● -C=C- Stretch =C-H Stretch ● ● 1680-1640 3100-3000 ● ● 1653.05 3022.94 Discussion Questions: 1. Our percent yield of cyclohexane was low at 37.98%. This can be explained by a multitude of things. For example, when placing the cleaned cyclohexane into the vial some was spilt out onto the countertop. Another reason could be that when we were distilling the liquid, we didn’t take the reaction flask as far as it could have gone. 2. 3. The range of the experimental boiling point for cyclohexene was 74°C to 80°C. The accepted boiling point range for cyclohexene is 82.9°C to 83°C, indicating that our sample is not completely pure. This error could have been caused by human error in distilling the solution. In the lab manual, it instruct the lab group to remove the receiving flask when the temperature reaches 80°C and attach a new one because the fraction collected between 80°C and room temperature was not cyclohexene. The lab group did have a small amount of liquid distilled between room temperature and 80°C so a small part of the fraction collect was impure. 4. The main difference between cyclohexanol and cyclohexene was a peak of 3325.30 cm-1 present in they cyclohexanol, but not in the cyclohexene. This peak is related to the alcohol group found in cyclohexanol. Both the cyclohexanol and the cyclohexene had mostly similar peaks. Overall, since the cyclohexene did not have a peak at 3325.30 cm-1 this provides evidence that cyclohexene doesn’t contain any impurities. 5. When the solution was mixed with Br2 the solution remained clear and colorless which proves that the bromine reacted with the alkene to form colorless dibromide. If the solution contained any impurities then the solution would have turned a reddish-brown color. Conclusion: In conclusion, we were successfully able to turn cyclohexanol into cyclohexene via a dehydration reaction. We found that the limiting reagent was cyclohexanol because it has the least amount of product in the reaction. By using a distillation setup we were able to completely change the 10 mL of cyclohexanol to cyclohexene the main purpose of the reaction. We then washed the substance with 5 mL of 10% sodium carbonate, 5 ml of water, and then finally 5 ml of saturated NaCl. After this, we used calcium chloride to dry this solution. After a week of drying, we perform another distillation to remove the crude cyclohexene from the water after the drying process. We found that the boiling range was from 74°C to 80°C meaning our solution was not completely pure. After cooling we placed a sample of our acquired liquid and found that the OH peak had completely disappeared meaning we did successfully convert the alcohol to an alkene. Elexis Farrell x Ashley Howes x Amrita Kapat x