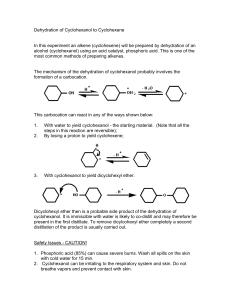
Reactions of metals and their compounds
advertisement
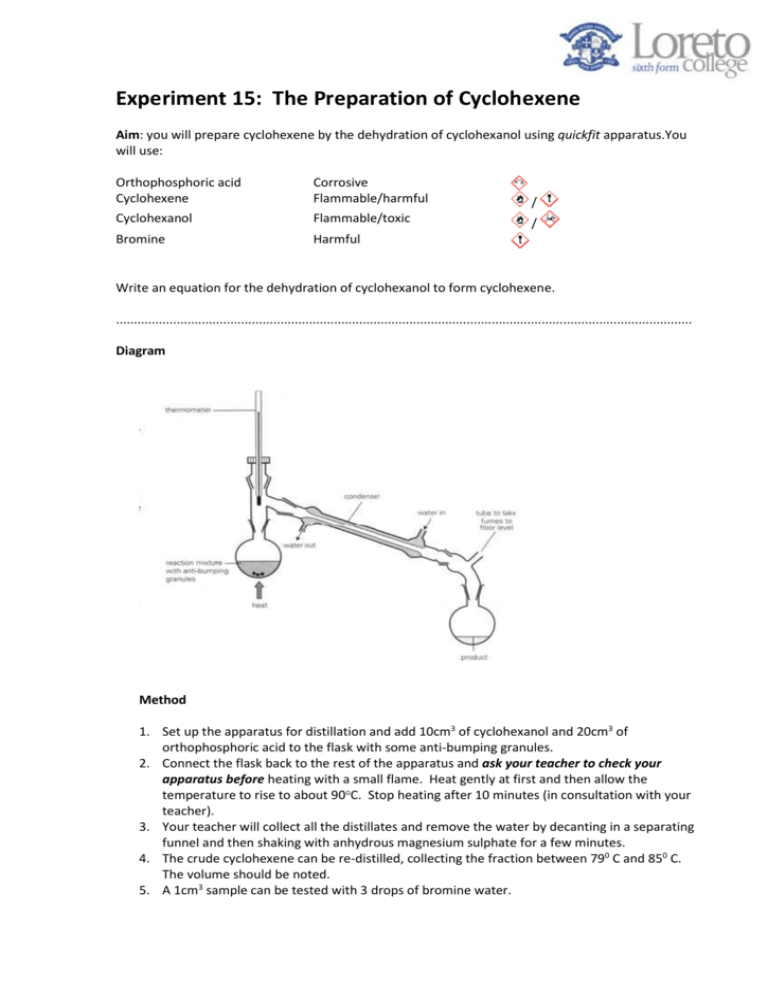
Experiment 15: The Preparation of Cyclohexene Aim: you will prepare cyclohexene by the dehydration of cyclohexanol using quickfit apparatus.You will use: Orthophosphoric acid Cyclohexene Corrosive Flammable/harmful / Cyclohexanol Flammable/toxic / Bromine Harmful Write an equation for the dehydration of cyclohexanol to form cyclohexene. ................................................................................................................................................................. Diagram Method 1. Set up the apparatus for distillation and add 10cm3 of cyclohexanol and 20cm3 of orthophosphoric acid to the flask with some anti-bumping granules. 2. Connect the flask back to the rest of the apparatus and ask your teacher to check your apparatus before heating with a small flame. Heat gently at first and then allow the temperature to rise to about 90 C. Stop heating after 10 minutes (in consultation with your teacher). 3. Your teacher will collect all the distillates and remove the water by decanting in a separating funnel and then shaking with anhydrous magnesium sulphate for a few minutes. 4. The crude cyclohexene can be re-distilled, collecting the fraction between 790 C and 850 C. The volume should be noted. 5. A 1cm3 sample can be tested with 3 drops of bromine water. Evaluation questions: you must show working out for all numerical answer questions. 1. Which layer is your organic compound in? Explain your answer. …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… 2. Explain why an anhydrous magnesium sulphate is used as a drying agent. …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… 3. A thermometer is used in this apparatus set up. a. What would happen if the student forgot to put the thermometer in? …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… b. Give one experimental advantage of using a thermometer over a bung. …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… 4. Another student repeated this experiment and obtained 8g of cyclohexene. Compound Cyclohexanol Cyclohexene Water Formula C6H11OH C6H10 H2O Mr 100 82 18 a) Calculate the theoretical yield for this reaction …………………… g b) Calculate the percentage yield for this reaction. Give your answer to 1 dp. …………………… % c) Calculate the atom economy for the reaction. Give your answer to 1 dp. …………………… % 5. Another method for producing cyclohexene is shown below: Give one possible advantage and one possible disadvantage for using this reaction to produce cyclohexene compared to the dehydration of cyclohexanol. Advantage: ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… Disadvantage: …………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… Extension Questions: What effect would adding too much cyclohexanol have on your calculated yield and why? …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… What effect would not drying the cyclohexene product with calcium chloride have on your calculated yield and why? …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… What effect would adding a smaller amount of orthophosphoric acid might have on your yield and why? …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………………………………