Cyclohexene from Cyclohexanol
advertisement

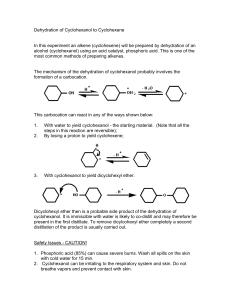
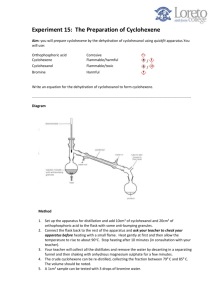
Cyclohexene from Cyclohexanol Introduction: The purpose of this experiment is to synthesize cyclohexene from cyclohexanol, this is accomplished through the elimination of the OH functionality from cyclohexanol by an acid (sulfuric or phosphoric) to yield cyclohexene and water; The intermediate step of this reaction yields a carbocation that can either react with water to form the starting material cyclohexanol; can lose a proton to yield cyclohexene; It is likely that dicyclohexyl ether is a side product of this reaction. Techniques such as infrared spectroscopy and H NMR spectroscopy can be utilized to identify the product in addition to measuring melting points. Results: Mechanism: Physical Properties: Safety: 1. Phosphoric acid (85%) can cause severe burns. Wash all spills on the skin with cold water for 15 min. See your demonstrator. 2. Bromine causes severe burns. Keep bromine away from your skin. Do not breathe the vapours. Use only in the FUME HOOD. 3. Cyclohexanol can be irritating to the respiratory system and skin. Do not breathe vapours and prevent contact with skin. Discussion: After fractional distillation is setup, 10.0g of Cyclohexanol and 3 ml ofphosphoric acid are gently heated in the round bottom flask with boiling chips, the distillation flask is placed in an ice bath; The reaction is stopped when white fumes can be observed in the reaction vessel and approximately 10 ml of the distillate have been collected. The product is then transferred to a separatory funnel where it will separate into two layers. The lower layer is then discarded. Wash the remaining layer (cyclohexene) with 10 ml of water and then separate the layers by letting the funnel run. The cylohexene is washed again with 10 ml of 10% sodium carbonate solution and the process of separation is repeated. The cyclohexene is washed again with water, separated and then transfered to another flask. Calcium chloride is an effective drying agent and is then added to the cylcohexene. The mixture is then left to stand for roughly 15 minutes and then gravity filtered leaving the cyclohexene product. Reference: 1. University of Massachusetts Amherst, Synthesis of Cyclohexene The Dehydration of Cyclohexanol, www.chem.umass.edu/~samal/269/cyclohexene.pdf 2. University of the West Indies, Preparation of cyclohexene from cyclohexanol, wwwchem.uwimona.edu.jm/lab_manuals/c10expt8.html