Name: __________________________________ Date: ________________ Class: __________
advertisement

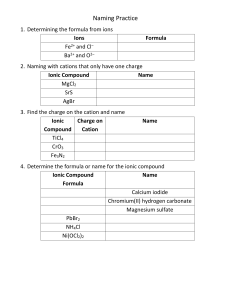
Name: __________________________________ Date: ________________ Class: __________ Define the following terms, using your notes and textbook readings (try not to use the internet): Ionic Bond Molecular element Hydrate Formula mass family Covalent Bond Molecular compound Acid Mass percent composition (mass percent) Alkane Ionic compound Binary acid Empirical formula molar mass Alkene Formula unit Oxyacid Combustion analysis Alkyne Products Polyatomic ion Balanced chemical reaction Chemical reaction Functional group Oxyanion Common name Hydrocarbon Reactants alcohol Systematic name Chemical formula Empirical formula Molecular formula Structural formula Ball-and-stick model Binary compound Space-filling molecular model Atomic element 1. What is the difference between ionic and covalent bonding? How do ionic bonds form (be specific and reference valence electrons and/or charges). 2. Compare and contrast empirical and molecular formulas. 3. What is the difference between the three types of organic molecules? What must be present to be considered organic? 4. What is a functional group? What are the 7 groups discussed? Draw an example of each. 5. What are formulas for the following: a. Formula Mass b. Mass Percent Composition c. Empirical Formula Molar Mass 6. Complete the following questions from your textbooks. Use the list to cross out your progress. Page 121-129, #1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 15, 17, 18, 19, 20, 21, 22, 23, 24, 27, 28, 29, 30, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 65, 66, 73, 74, 75, 77, 79, 80, 81, 85, 86, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 105, 106, 107, 108 Unit 3