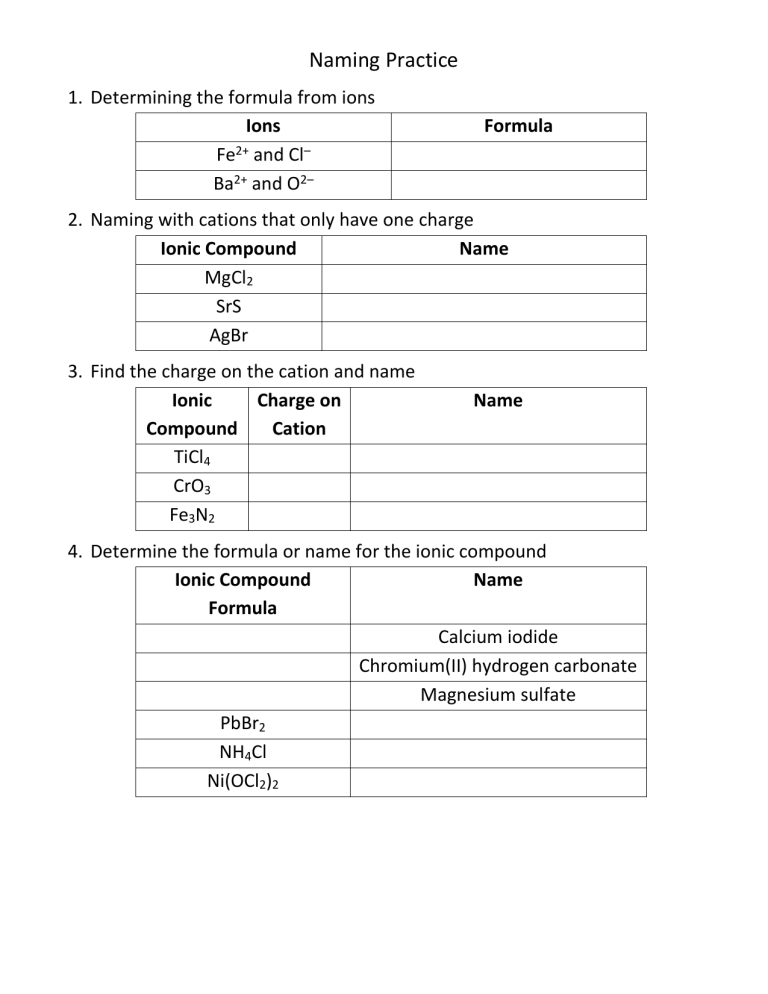
Naming Practice 1. Determining the formula from ions Ions Fe2+ and Cl– Ba2+ and O2– Formula 2. Naming with cations that only have one charge Ionic Compound Name MgCl2 SrS AgBr 3. Find the charge on the cation and name Ionic Charge on Compound Cation TiCl4 CrO3 Fe3N2 Name 4. Determine the formula or name for the ionic compound Ionic Compound Name Formula Calcium iodide Chromium(II) hydrogen carbonate Magnesium sulfate PbBr2 NH4Cl Ni(OCl2)2 Naming Practice 5. Determine the formula or name for the molecular compound Molecular Compound Name Formula Dinitrogen tetroxide Sulfur hexafluoride Carbon dioxide PCl5 XeF4 IF7 6. Identify compounds as Ionic or Molecular Compound Ionic or Molecular? MgCl2 NaOH H2S Ca3(PO4)2 IF7 SrS PH3 AgBr NH4Cl 7. Determine the formula or name for the acid Acid Formula Name Chlorous acid Phosphoric acid Hydrobromic acid H2CO3 H2SO3 HF






