AP Chemistry Podcast 1.2 Calculating Empirical and Molecular
advertisement

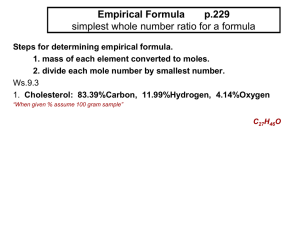
AP Chemistry Podcast 1.2 Calculating Percent Yield, Percent Composition, Percent Error, Empirical and Molecular Formulas Percent Yield Rules 1. Balanced Equation 2. Write amount given under the reactant that has a value 3. You will be given an amount of product (write this under the product) this is the actual amount produced. 4. Convert given reactant amount to same product that was given. 5. Percent Yield = Example: Percent Yield Nitrogen gas reacts with hydrogen gas to make ammonia (NH3 ). 15.5 L of N2 reacts at STP to make 30 L of ammonia. What is the percentage yield? Percent Composition • Find the percentage composition of a chemical by finding the total molar mass of the compound…find the mass percent of each element in the compound. • Example: NH4NO3 Percent Error Empirical Formula: Rules 1. Percent to mass 2. Mass to moles 3. Divide by small 4. Times to whole Example: Empirical Formula Determine the empirical formula of a compound with 52.8% Sn, 12.4% Fe, 16% C, and 18.8% N. Molecular Formulas 1. Molecular formulas are the actual number of atom’s in a molecule…not just the simplest whole number ratio. 2. The molar mass of the empirical formula is always a multiple of the molecular weight. 3. Use the molecular weight to determine what factor you need to multiple the empirical formula to find the molar formula. Example: Molecular Formula Determine the molecular formula for a compound that contains 22.5% Na, 30.4% P and 47.1%O and a molar mass of 306 g/mol.