Topic: Molecular Compounds
advertisement

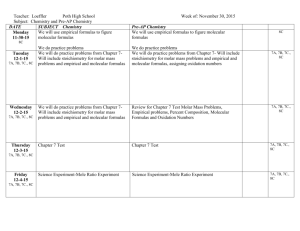
Name ______________________________________ Topic: Chemical Quantities Key Learning(s): The mole is used to convert between mass. Percent composition can be used to identify a compounds empirical and molecular formulas. Unit Essential Question(s): What information can be derived from the chemical formula of a compound? Concept 1: Mass Conversions 3.2.C.A.2: Use the mole concept to determine number of particles and molar mass for elements and compounds. Lesson Essential Questions: How do you calculate molar mass? Vocabulary: 1. Mole 2. Molar Mass Period _______ Subject: Chemistry Grade(s): 10, 11, & 12 Instructional Tools: PH chapter 10 Graphic Organizers Worksheets Lab Concept 2: Percent Composition 3.2.C.A.2: Determine percent compositions, empirical formulas, and molecular formulas. Concept 3: Empirical & Molecular Formulas 3.2.C.A.2: Determine percent compositions, empirical formulas, and molecular formulas. Lesson Essential Questions: How do you calculate percent by mass of an element in a compound? Lesson Essential Questions: What is the difference between empirical and molecular formulas and how can they be determined? Vocabulary: 3. Percent Composition Vocabulary: 4. Empirical Formula 5. Molecular Formula Name ______________________________________ Period _______