Chemistry Worksheet: Diatomic Molecules, Formulas, Equations
advertisement

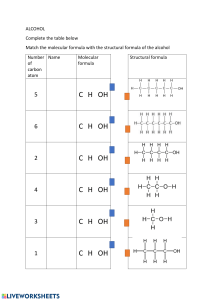
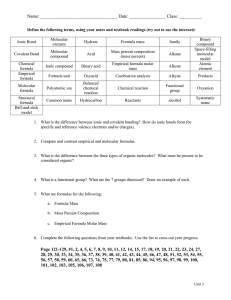
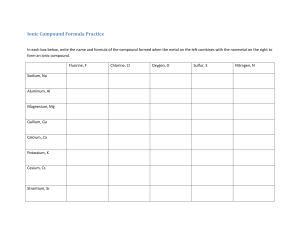
Chapter 3 Worksheet 1. List the 7 diatomic molecules 2. Determine if the following are atomic element, molecular element, molecular compound, or ionic compound a. H2O b. Pb c. PbO2 d. F2 3. Fill in the table Name Formula Molecular or Ionic Diboron hexahydride BaSO4 Manganese (IV) oxide CCl4 Ti(ClO4)2 C3H8 4. Predict the charge and formula for the following ionic compounds a. Rubidium and oxygen b. Iron (III) and nitrate c. Zinc and carbonate d. Strontium and chlorine e. Ammonium and hydroxide 5. How many moles are in 35.3 g NaOH? How many formula units? 6. How many milligrams are in 2.45 x 1023 molecules of water? 7. How many fluorine atoms are in 5.6 grams of sulfur hexafluoride? 8. (a) Determine the mass of oxygen in a 7.2 g sample of Al2(SO4)3. (b). How many sulfur atoms are in 45.0 µg of Al2(SO4)3? 9. What is the mass percent of chlorine in calcium chlorate? 10. A compound with the percent composition of 39.97 % C, 13.41 % H, and 46.62 % N has a molar mass of 60.10 g/mol. What is the empirical and molecular formula? 11. When iron rusts, solid iron reacts with gaseous oxygen to form solid iron (III) oxide. Write a balance equation for this reaction. 12. Balance each chemical equation a. ______Co(NO3)3 + _________(NH4)2S _______Co2S3 + ________NH4(NO3) b. _______N2H4 ________NH3 + ________N2 c. _______FeS + _______HCl ________FeCl2 +_______ H2S