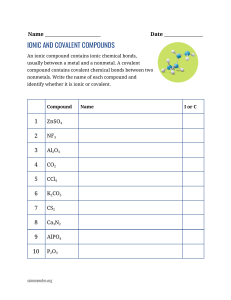
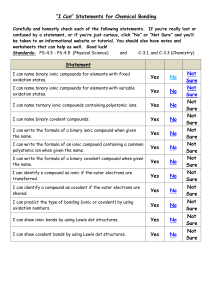
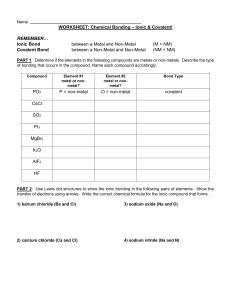
Ionic and Molecular Bonds Worksheet
advertisement
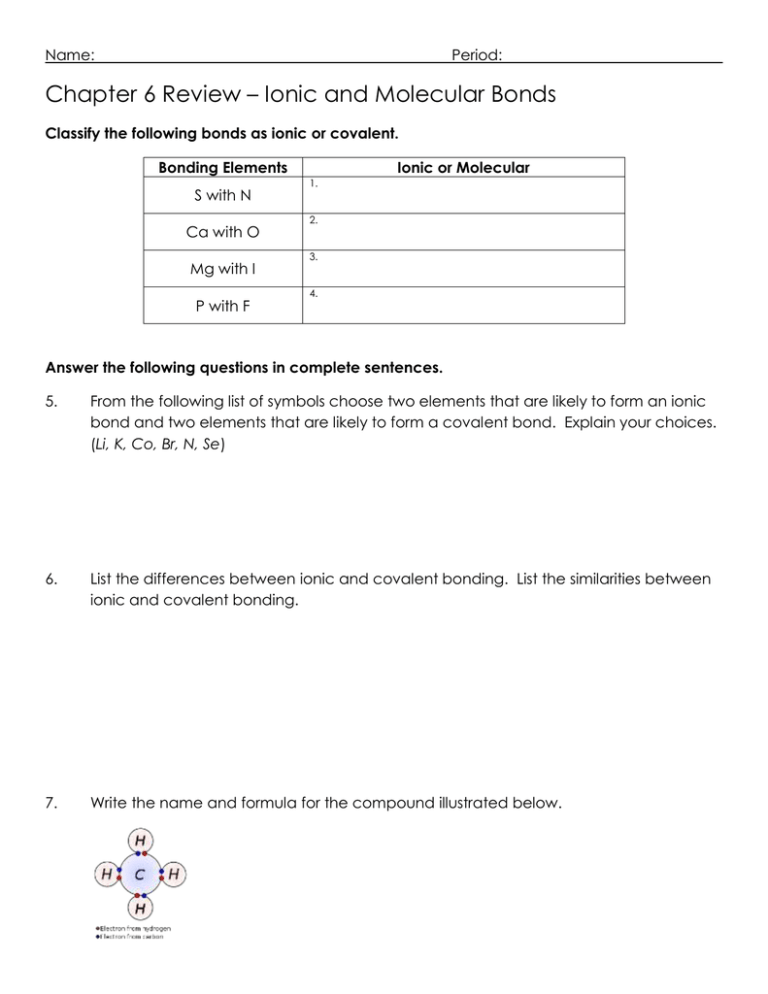
Name: Period: Chapter 6 Review – Ionic and Molecular Bonds Classify the following bonds as ionic or covalent. Bonding Elements S with N Ca with O Mg with I P with F Ionic or Molecular 1. 2. 3. 4. Answer the following questions in complete sentences. 5. From the following list of symbols choose two elements that are likely to form an ionic bond and two elements that are likely to form a covalent bond. Explain your choices. (Li, K, Co, Br, N, Se) 6. List the differences between ionic and covalent bonding. List the similarities between ionic and covalent bonding. 7. Write the name and formula for the compound illustrated below. 8. What processes change atoms to cations and anions? Write formulas for the following compounds/molecules. Compound Formula 9. Sodium oxide 10. Strontium fluoride 11. Ammonium phosphate 12. Trinitrogen tetroxide 13. Copper (IV) sulfide Write the name of the following compounds/molecules. Compound Formula LiF K3P N2S5 Pb2SO4 H2O Compound Name 14. 15. 16. 17. 18.