What coefficient is needed to balance this equation? Explain • 2H
advertisement

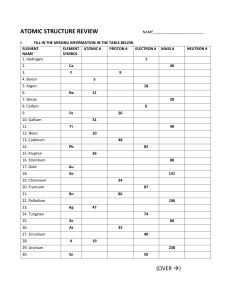
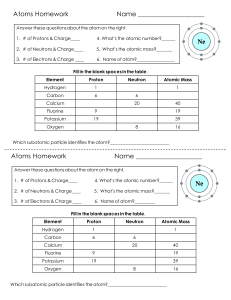
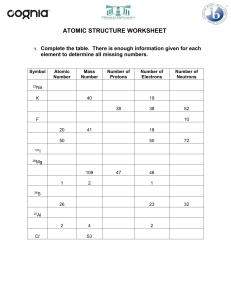
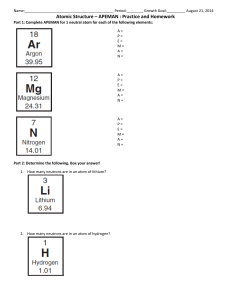
Question 1 • 2H2 + O2 H2O What coefficient is needed to balance this equation? Explain Question 2 • 5CH4 + 3O2 CO2 + 3H2 O How many molecules are on the reactants side? Explain Question 3 Write this down as a chemical equation. Question 4 •Explain the Law of Conservation of Mass. Question 5 Why is this chemical equation balanced? Question 6 •Why is the atomic number of lead 82? Question 7 •Why does the Chlorine atom have 18 neutrons? Question 8 •How many atoms are in CO ? 2 Question 9 • If an atom is balanced, and it has an atomic number of 26 and an atomic mass of 55.847. How many electrons are in this atom. Question 10 •Calculate how many neutrons Argon has. Question 11 •What period is Calcium on? Question 12 •Why is Hydrogen more reactive than Helium? Question 13 •How do you know Zirconium is a metal? Question 14 •What are the two names of the vertical columns on the Periodic Table? Question 15 What is this number called? Question 16 Question 17 Question 18 Question 19 Question 20 Question 21 Question 22 Question 23 Question 24 Question 25 Question 26 Question 27 Question 28 Question 29 Question 30