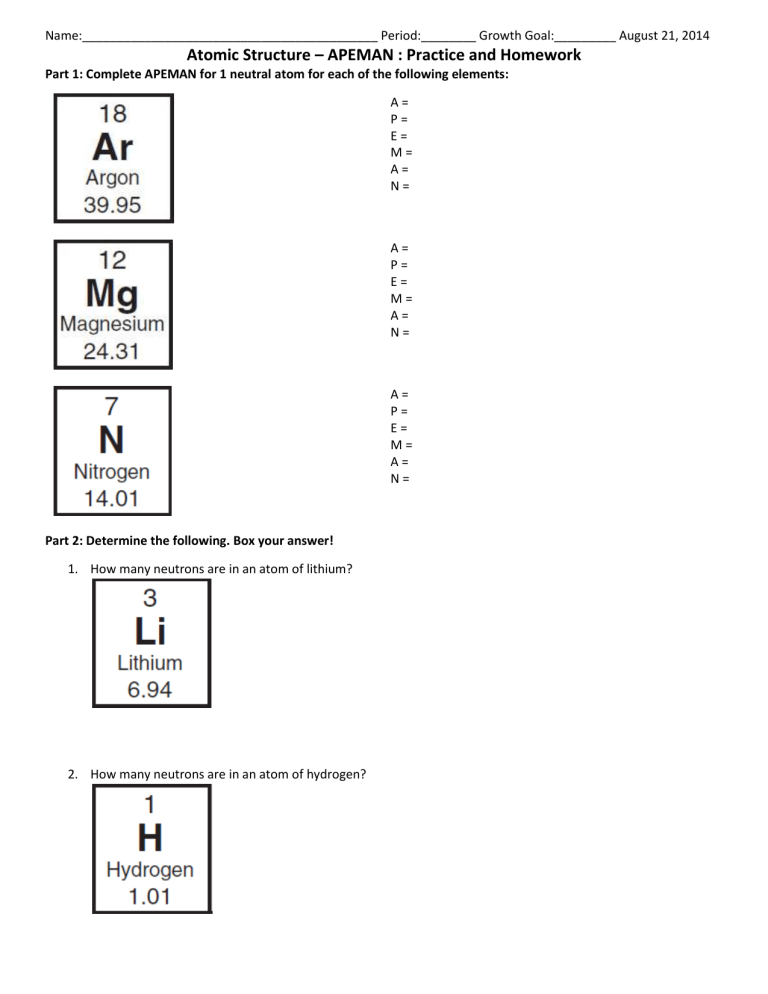
Name:___________________________________________ Period:________ Growth Goal:_________ August 21, 2014 Atomic Structure – APEMAN : Practice and Homework Part 1: Complete APEMAN for 1 neutral atom for each of the following elements: A= P= E= M= A= N= A= P= E= M= A= N= A= P= E= M= A= N= Part 2: Determine the following. Box your answer! 1. How many neutrons are in an atom of lithium? 2. How many neutrons are in an atom of hydrogen? Name:___________________________________________ Period:________ Growth Goal:_________ August 21, 2014 Atomic Structure – APEMAN : Practice and Homework 3. In a neutral atom, this element has an atomic mass of 23 AMU and has 11 protons. a. How many neutrons does it have? ____________________ b. What element is it ? ______________________________ 4. In a neutral atom, this element has 5 neutrons and an atomic number of 5. a. What is the atomic mass? ______________________ b. What element is it? ____________________ 5. In a neutral atom, this element has 16 neutrons and 15 electrons. a. What is the atomic mass? ______________________ b. What element is it? ____________________ Part 3: Review 1. The atomic number of an element equals the number of ______________ in its atoms. 2. Label the atomic number, chemical symbol, and the atomic mass/mass number: o What is the atomic number of oxygen? ________________________ o What is the atomic mass of oxygen? ___________________________ o In one neutral atom of oxygen, there are _____ protons, _____ neutrons, and _____electrons. 3. Label the Table below Particle Electron Mass Electrical Charge Location in Atom Proton Neutron 4. Draw a diagram of an atom, labeling the two major areas of the atom and where the 3 subatomic particles can be found