atomicobjective6stand
advertisement

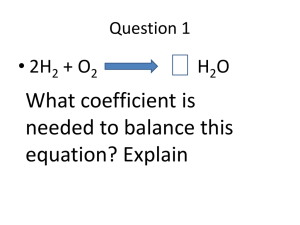
Atomic Structure 6 Objective: Students will be able to examine the parts of an atom in order to be able to demonstrate how to determine which groups of elements form cations and which form anions 1. What will we learn today? _____________________________________ _____________________________________________________________ 2. How will you learn this? ______________________________________ _____________________________________________________________ 3. List the parts of an atom and tell how you would determine them from information found in the periodic table of elements for each atom 4. Write the isotopic symbol for the following atoms IRON-56 + COPPER-66 + NITROGEN-16 + CARBON-14 5. Tell which of the above examples are isotopes of their atom, explain how you know. ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ 6. How could you determine the number of neutrons in an atom or an isotope? ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ 7. What is an ion? __________________________________________ _____________________________________________________________ Atomic Structure 6 8. Use the following isotope notation to fill in the blanks: 25 +2 12Mg Neutrons: __________ Atomic number: ______ Protons: ________ Mass Number: _______ Electrons: ________ Longhand Notation /Name: Is this an isotope Is this an: yes Cation or or no (circle your answer) Anion (circle your answer) 9. Use the periodic table to fill in the blanks about a neutral atom of Platinum . Protons: ________ Electrons: _________ Atomic number: _______ Average Atomic mass: ________ Average # Neutrons: __________ Draw Out the Isotopic Symbol Below